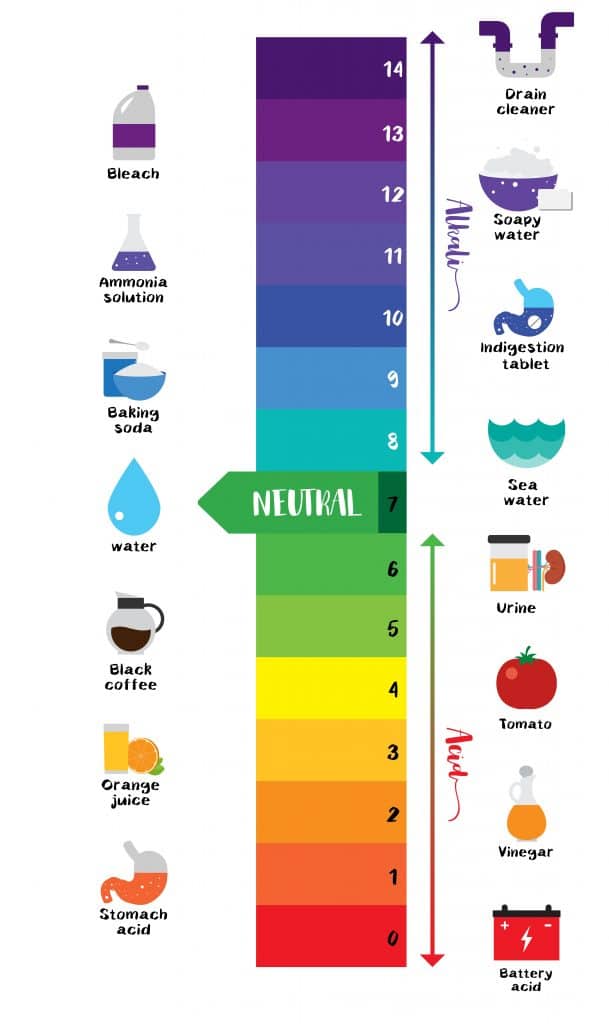
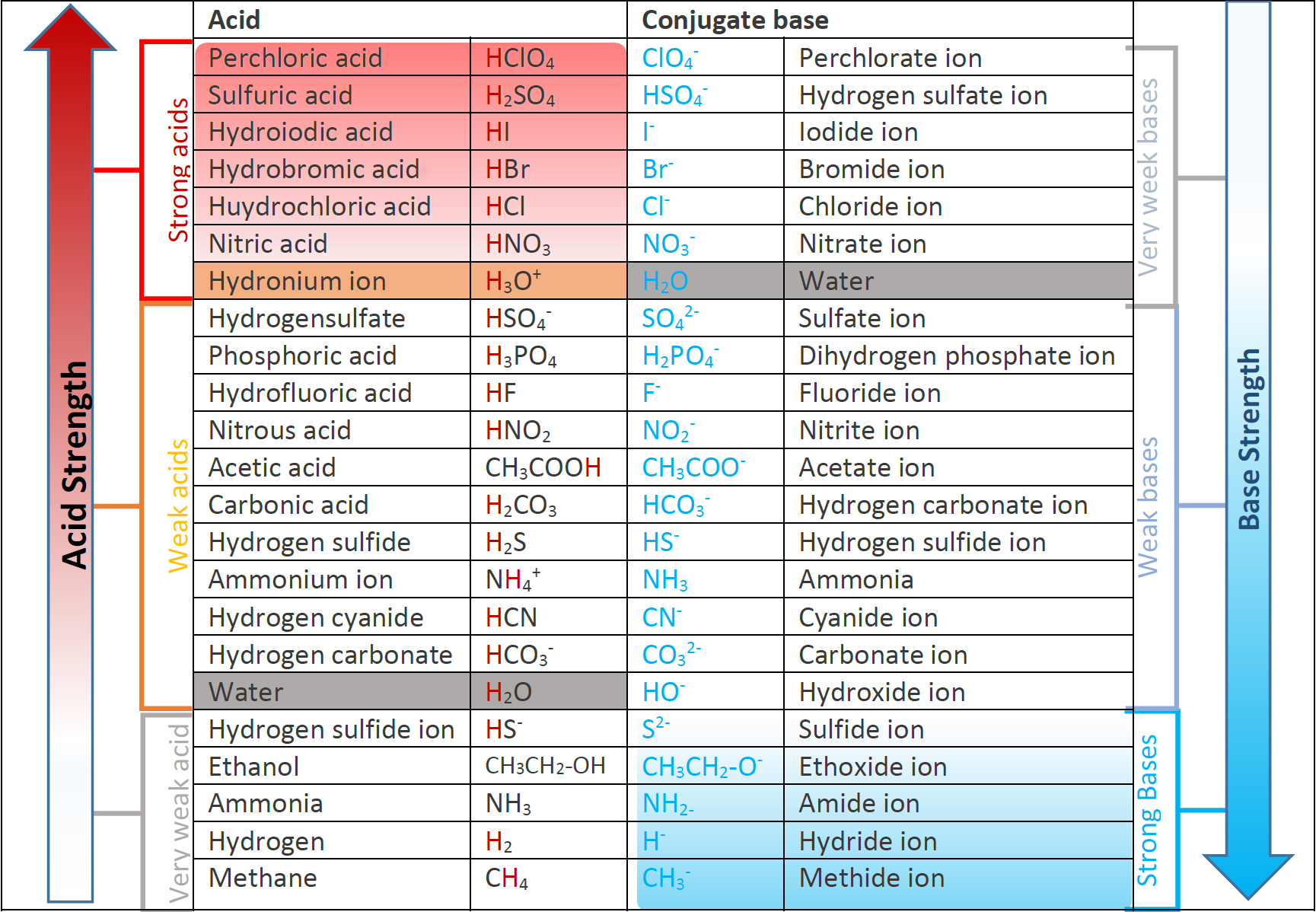
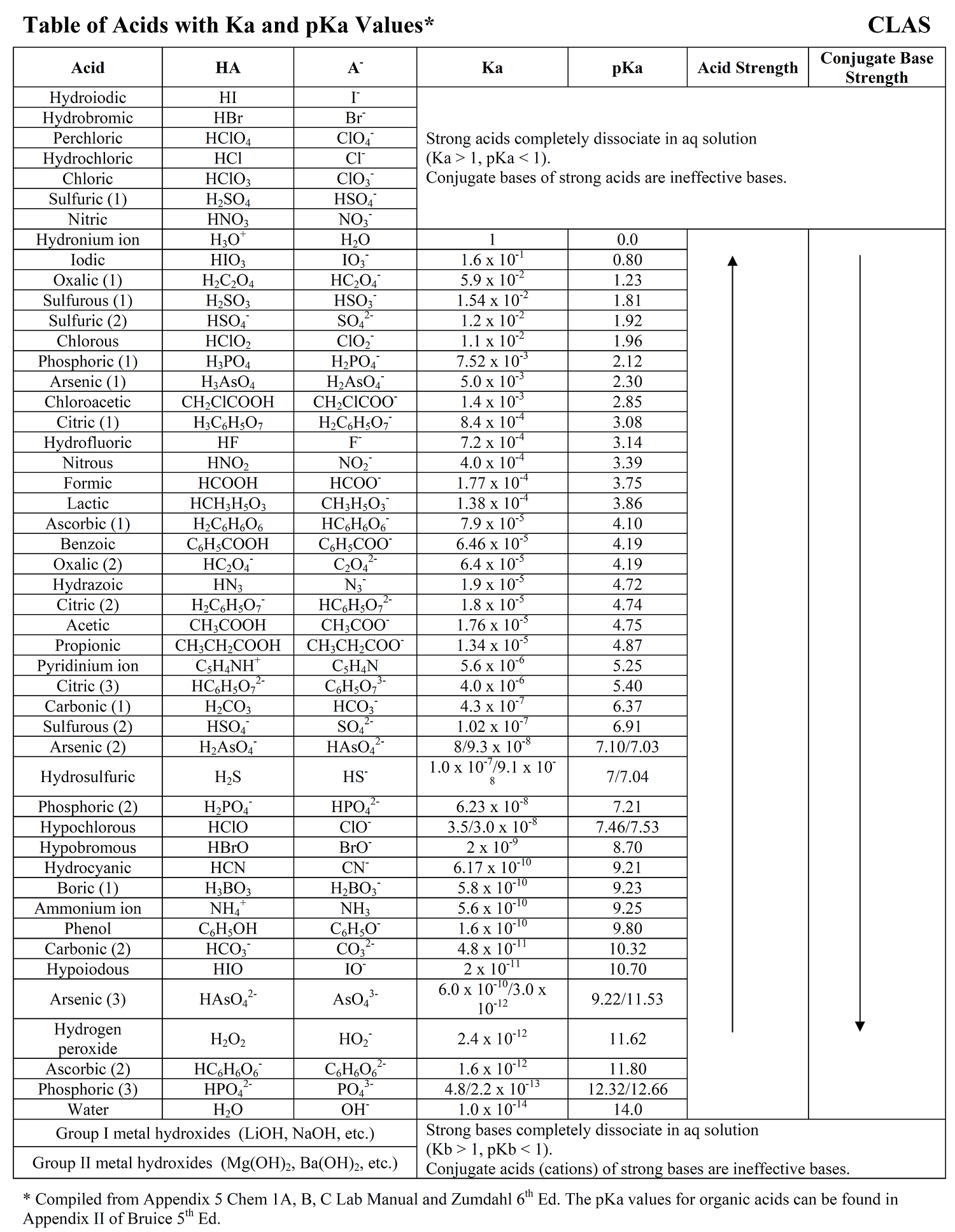
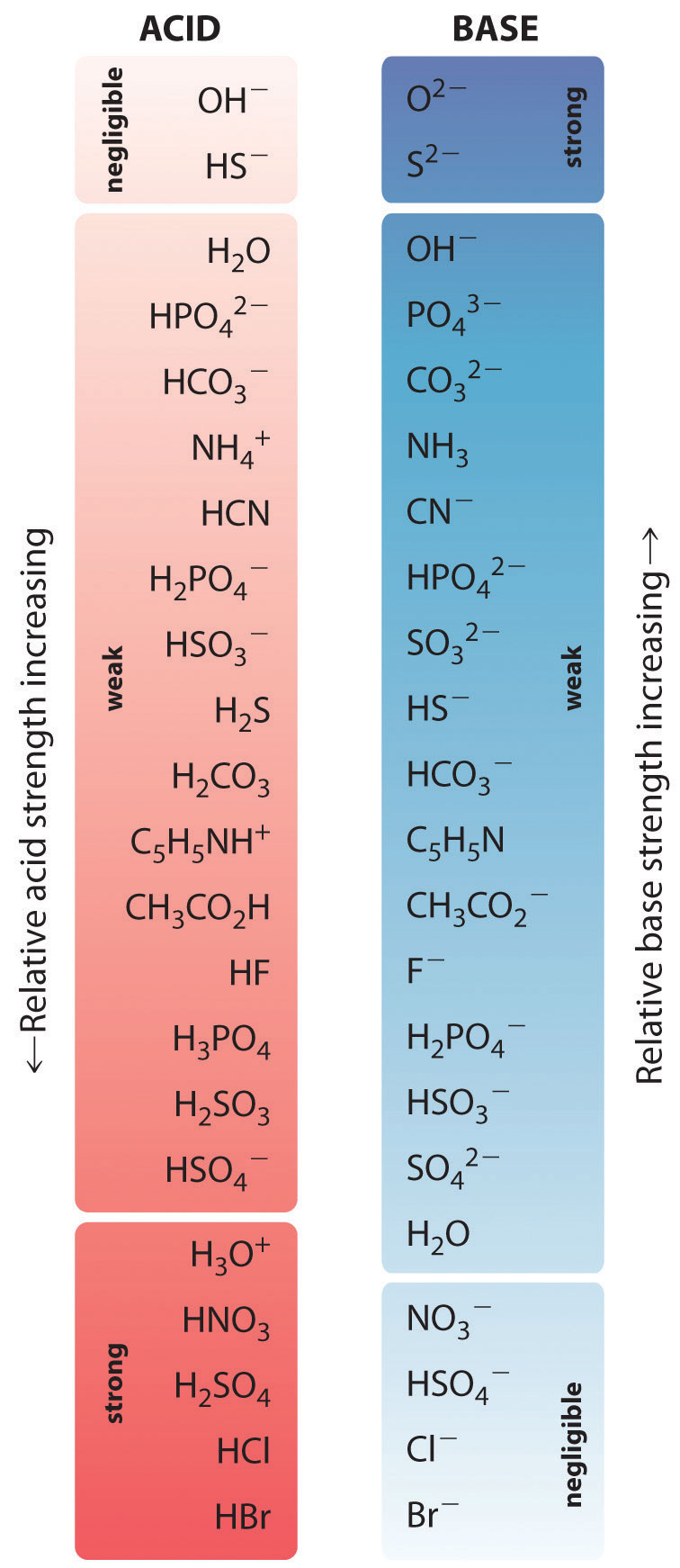
Acid Strength Chart
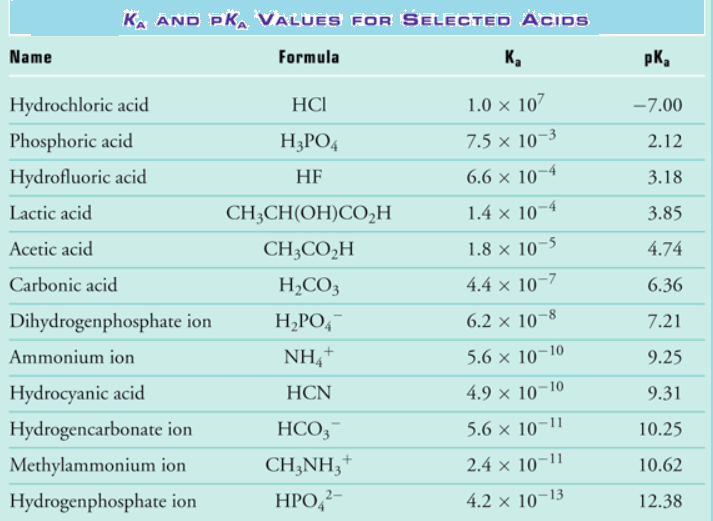
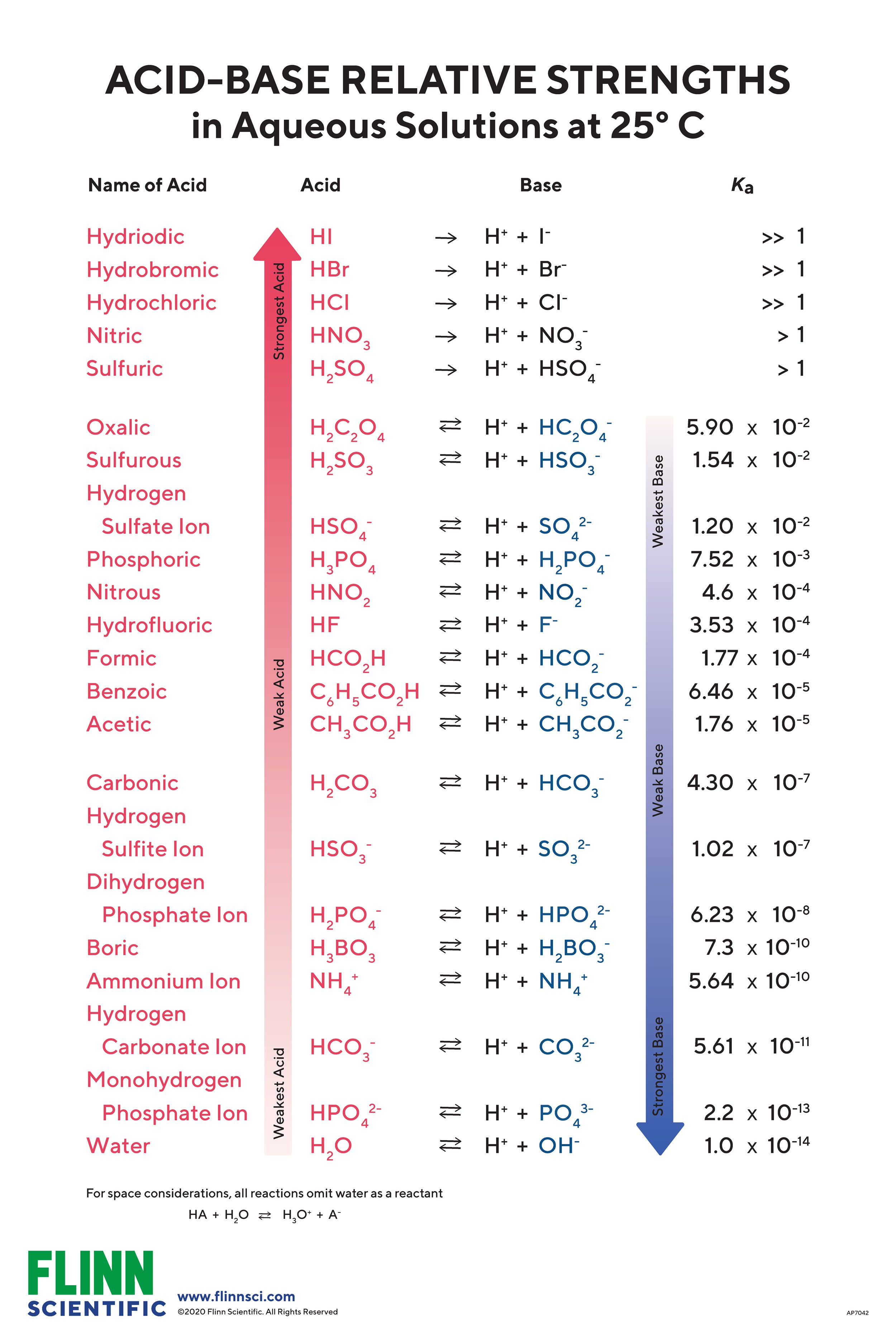
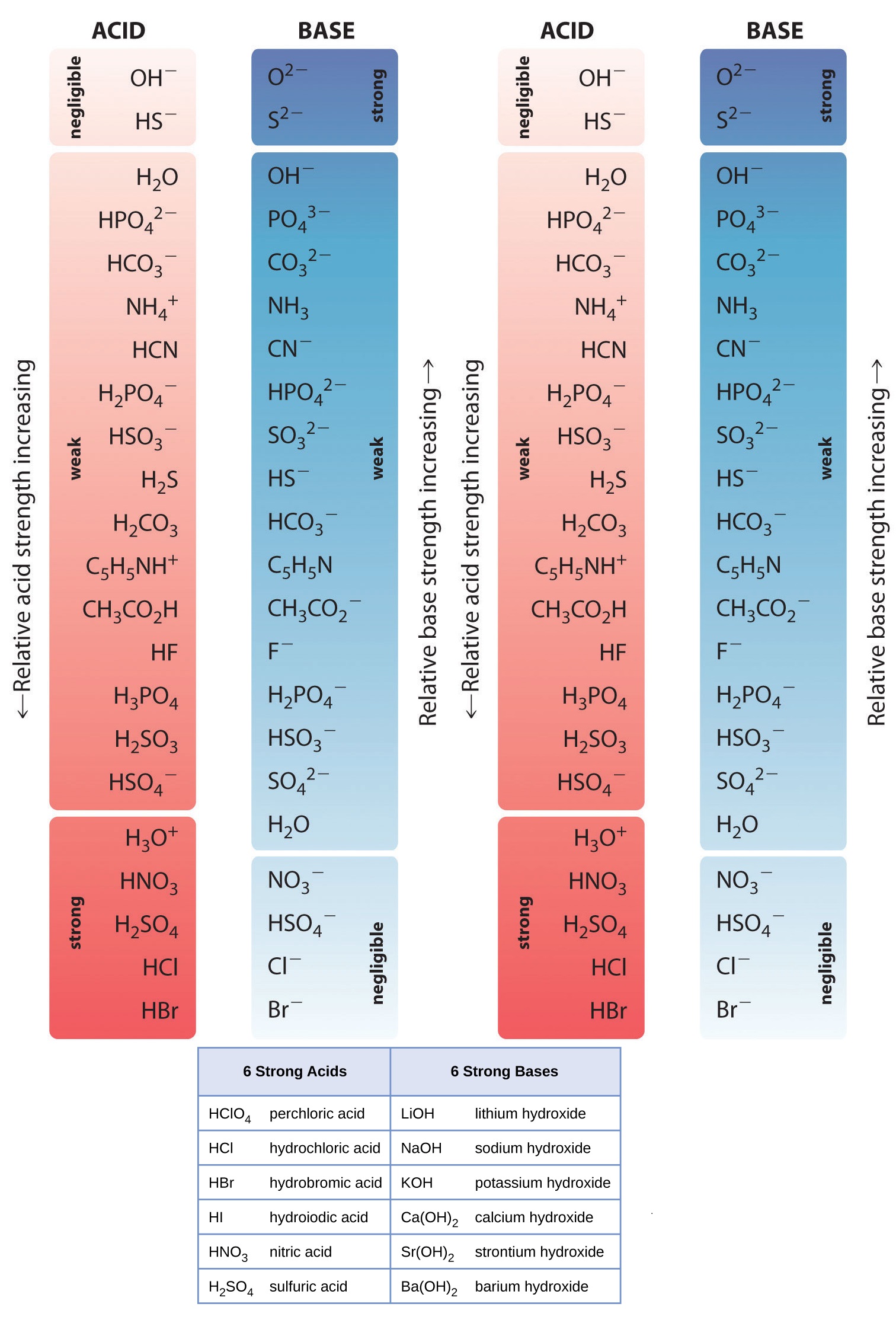
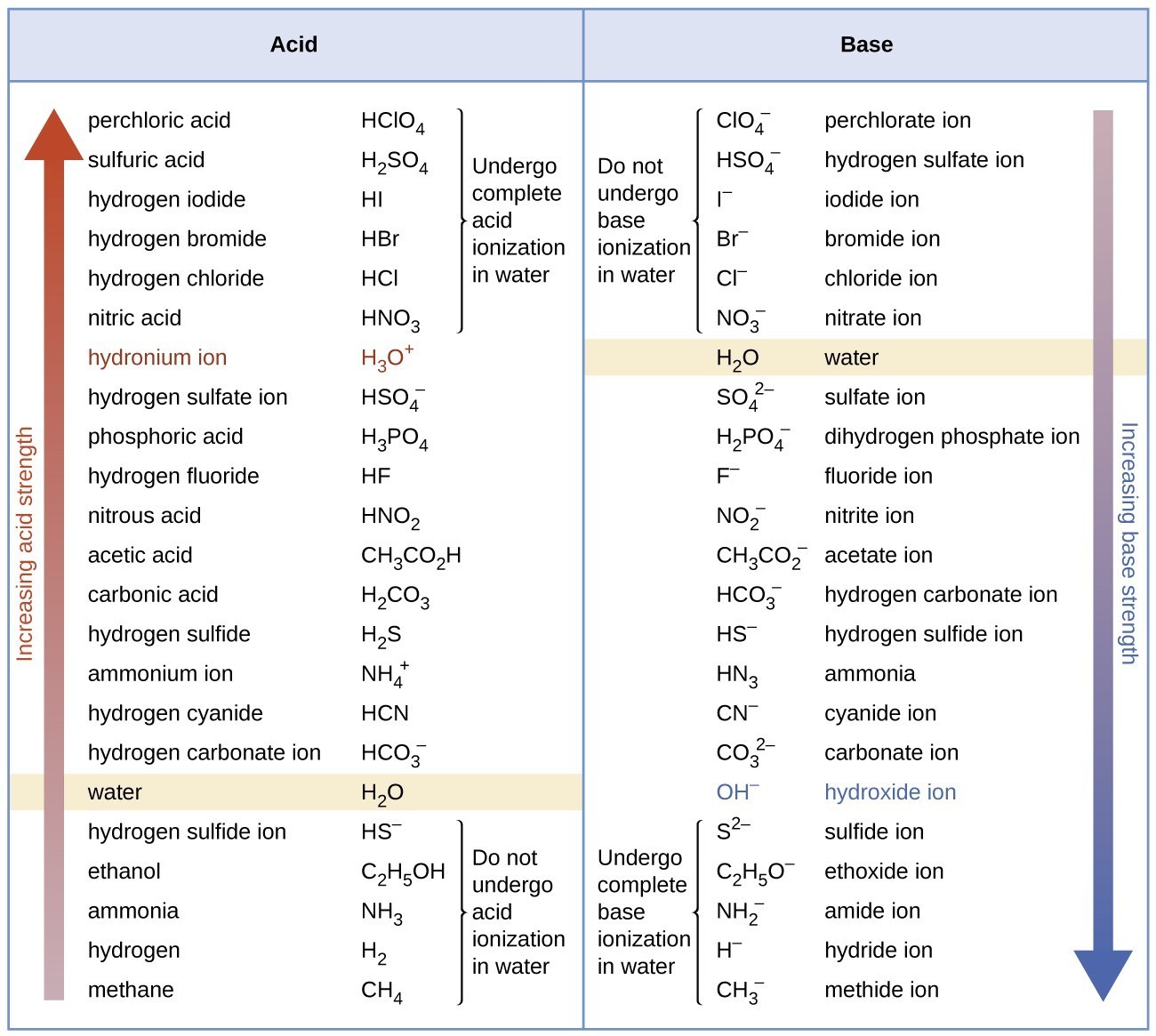
Acid Strength Chart - Web acid strengths are normally expressed using p ka values rather than ka values, where the pka is the negative common logarithm of the ka: Web definitions of ph, poh, and the ph scale. The first six acids in figure 15.3.3 are the most common strong acids. Web figure 15.3.3 lists a series of acids and bases in order of the decreasing strengths of the acids and the corresponding increasing strengths of the bases. Look at where the negative charge ends up in each conjugate base. Web a strong acid yields 100% (or very nearly so) of \(\ce{h3o+}\) and \(\ce{a^{−}}\) when the acid ionizes in water. The terms strong and weak describe the ability of acid and base solutions to conduct electricity. Web figure 14.3.3 lists a series of acids and bases in order of the decreasing strengths of the acids and the corresponding increasing strengths of the bases. Web the key to understanding this trend is to consider the hypothetical conjugate base in each case: They’re routinely described as strong or weak, concentrated or dilute. Calculating the ph of a strong acid or base solution. Web chart of acid and base strength. A weak acid does not completely ionize in water, yielding only small amounts of \(\ce{h3o+}\) and \(\ce{a^{−}}\). Web acid with values less than one are considered weak. Web use this acids and bases chart to find the relative strength of the most common acids and bases. The first six acids in figure 14.3.3 are the most common strong acids. Web the strength of acids and bases, i.e., the extent of dissociation of the dissolved acid or base into ions in water is described. For each acid, the ionization reaction shows the acid’s conjugate base. The relationship between acid strength and the ph of a solution. Web the relative strength of an acid or base is the extent to which it ionizes when dissolved in water. A stronger acid (larger ka) has a smaller p ka, and a. Web figure 15.3.3 lists a series of acids and bases in order of the decreasing strengths of the acids and the corresponding increasing strengths of the bases. The terms strong and weak describe the ability of acid and base solutions to conduct electricity. Even if you’re not a. If relatively little ionization occurs, the acid or base is weak. The first six acids in figure 15.3.3 are the most common strong acids. The terms strong and weak describe the ability of acid and base solutions to conduct electricity. A guide to acids, acid strength, and concentration. Web the relative strength of an acid or base is the extent. For each acid, the ionization reaction shows the acid’s conjugate base. This information can be used to predict the outcome of reactions between acids and other substances, such as bases and metals. If relatively little ionization occurs, the acid or base is weak. A weak acid does not completely ionize in water, yielding only small amounts of \(\ce{h3o+}\) and \(\ce{a^{−}}\).. Pka = −log ka p k a = − log k a. Web determine at a glance the relative strengths of a host of acids and bases. Web acid strengths are normally expressed using p ka values rather than ka values, where the pka is the negative common logarithm of the ka: The terms strong and weak describe the ability. Web definitions of ph, poh, and the ph scale. By andy brunning september 28, 2016. The first six acids in figure 15.3.3 are the most common strong acids. Web figure 14.3.3 lists a series of acids and bases in order of the decreasing strengths of the acids and the corresponding increasing strengths of the bases. Web use this acids and. If the ionization reaction is essentially complete, the acid or base is termed strong ; Table \(\pageindex{1}\) lists several strong acids. A stronger acid (larger ka) has a smaller p ka, and a. They’re routinely described as strong or weak, concentrated or dilute. A weak acid does not completely ionize in water, yielding only small amounts of \(\ce{h3o+}\) and \(\ce{a^{−}}\). The terms strong and weak describe the ability of acid and base solutions to conduct electricity. Web the relative strength of an acid or base is the extent to which it ionizes when dissolved in water. Web use this acids and bases chart to find the relative strength of the most common acids and bases. Calculating the ph of a. Even if you’re not a chemist, you’ll doubtless remember learning about acids back in school. Examples of strong acids are hydrochloric acid. Web the key to understanding this trend is to consider the hypothetical conjugate base in each case: Web use this acids and bases chart to find the relative strength of the most common acids and bases. The acid. If the ionization reaction is essentially complete, the acid or base is termed strong ; Web the strength of acids and bases, i.e., the extent of dissociation of the dissolved acid or base into ions in water is described. The acid and base in a given row are conjugate to each other. If relatively little ionization occurs, the acid or. Even if you’re not a chemist, you’ll doubtless remember learning about acids back in school. If relatively little ionization occurs, the acid or base is weak. Web use this acids and bases chart to find the relative strength of the most common acids and bases. Pka = −log ka p k a = − log k a. Table \(\pageindex{1}\) lists. Examples of strong acids are hydrochloric acid. Pka = −log ka p k a = − log k a. Web acid strength is the tendency of an acid, symbolised by the chemical formula, to dissociate into a proton, +, and an anion,. The more stable (weaker) the conjugate base, the stronger the acid. The terms strong and weak describe the ability of acid and base solutions to conduct electricity. Web use this acids and bases chart to find the relative strength of the most common acids and bases. This information can be used to predict the outcome of reactions between acids and other substances, such as bases and metals. Web the key to understanding this trend is to consider the hypothetical conjugate base in each case: The strong bases are listed at the bottom right of the table and get weaker as we move to the top of the table. If the acid or base conducts electricity strongly, it is a strong acid or base. Web use this acids and bases chart to find the relative strength of the most common acids and bases. By andy brunning september 28, 2016. A stronger acid (larger ka) has a smaller p ka, and a. The relationship between acid strength and the ph of a solution. Web the terms strong and weak give an indication of the strength of an acid or base. Web definitions of ph, poh, and the ph scale.Acid strength W3schools
AcidBase Strength Charts for Chemistry
List of Strong Acids & Bases in Order StudyPK
14.3 Relative Strengths of Acids and Bases Chemistry LibreTexts
Relative Strengths of Acids and Bases Chemistry Atoms First
Section 3 Strengths of Acids and Bases Nitty Gritty Science
6.3 Strength of acids and bases Chemistry LibreTexts
pKa Values and strengths of Acids and Bases
Acid Strength, Ka, and pKa Chemistry Steps
Acid Strengths Table
The First Six Acids In Figure 14.3.3 Are The Most Common Strong Acids.
For Each Acid, The Ionization Reaction Shows The Acid’s Conjugate Base.
A Guide To Acids, Acid Strength, And Concentration.
Web Figure 14.3.3 Lists A Series Of Acids And Bases In Order Of The Decreasing Strengths Of The Acids And The Corresponding Increasing Strengths Of The Bases.
Related Post: