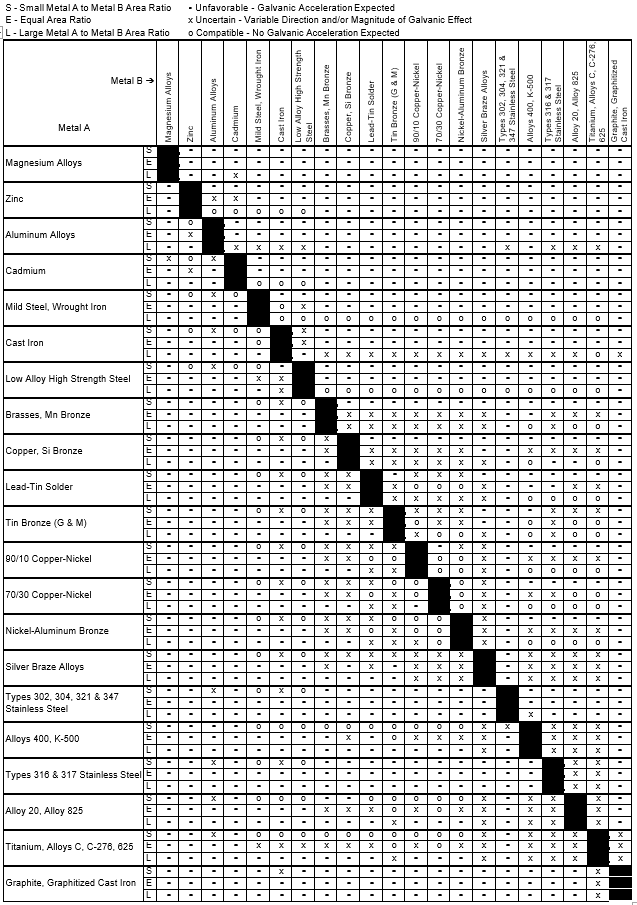
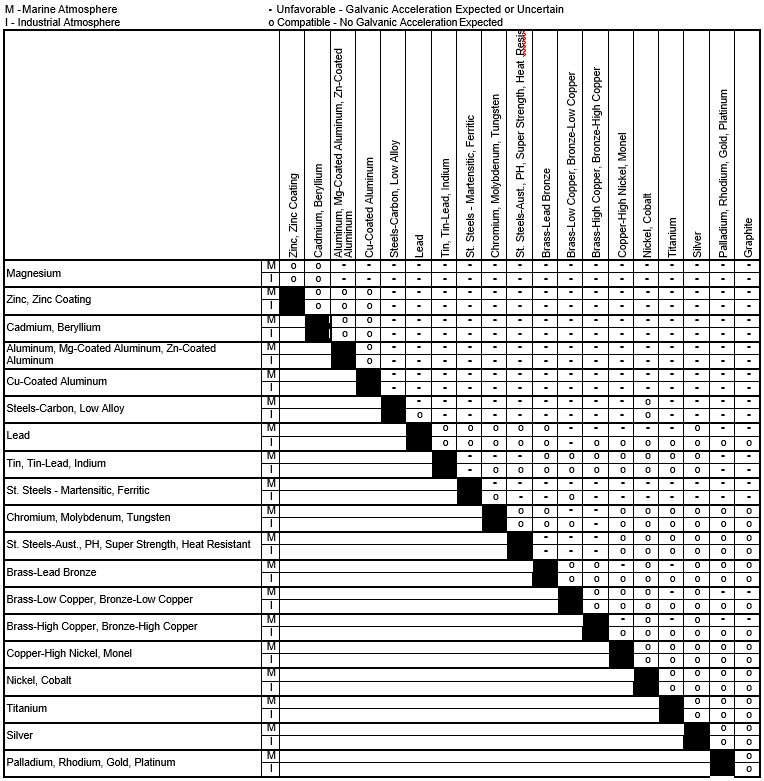
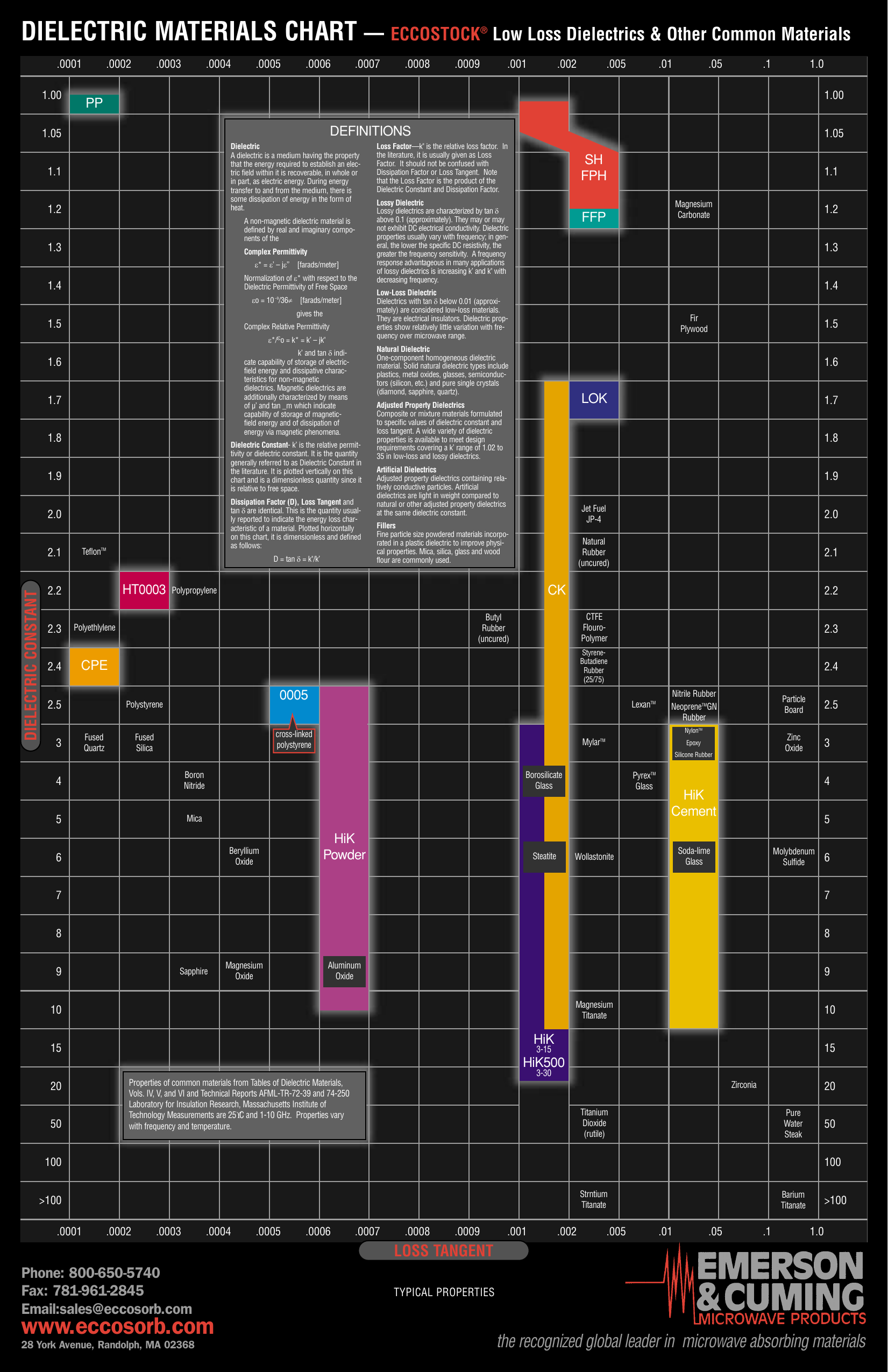
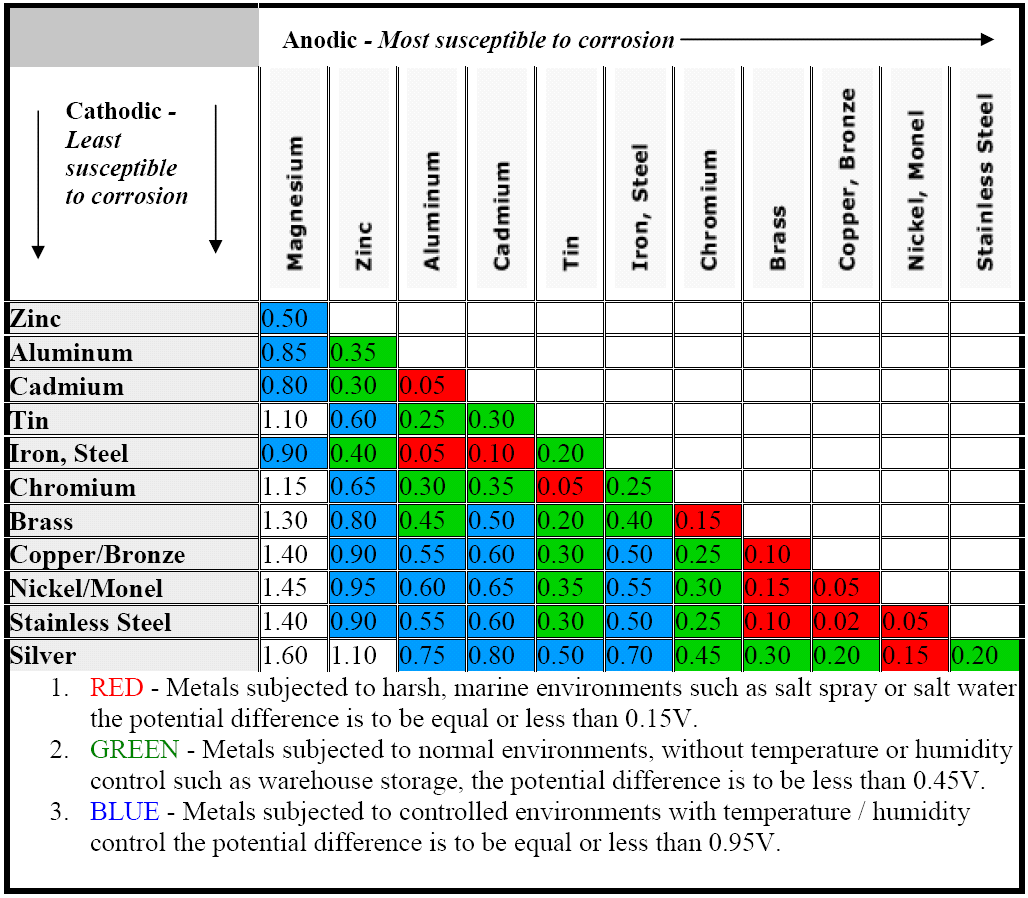
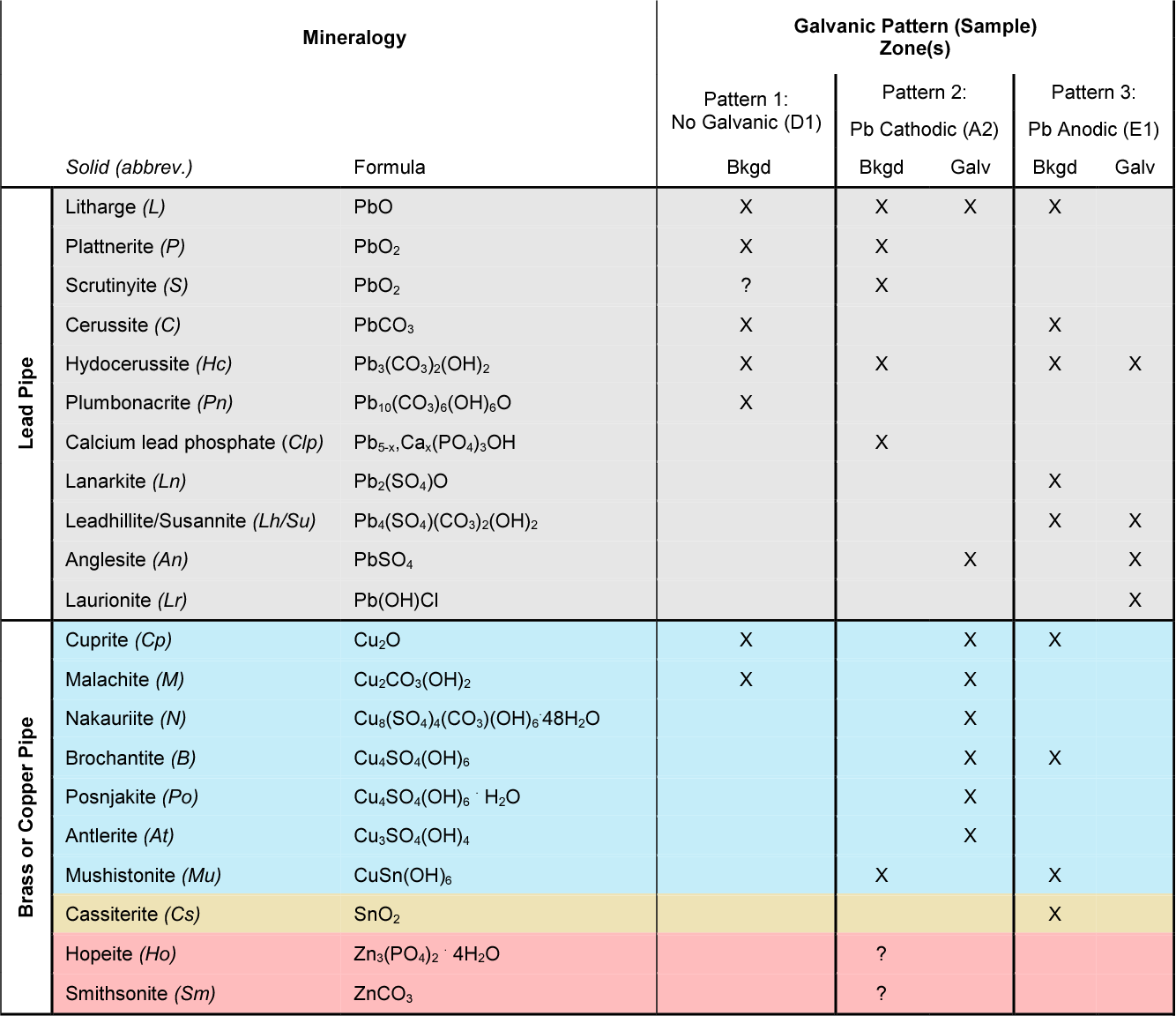
Dielectric Corrosion Chart
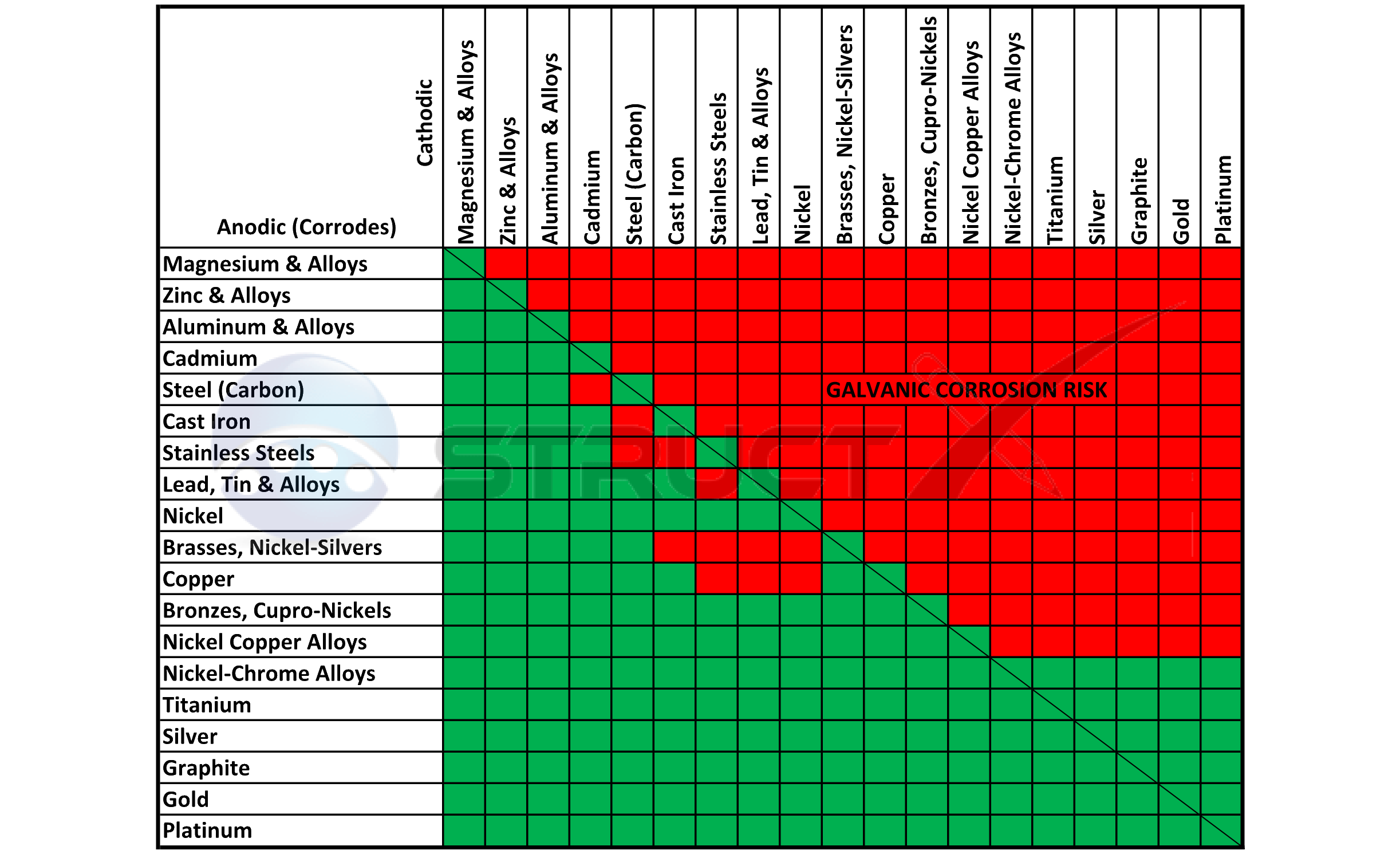
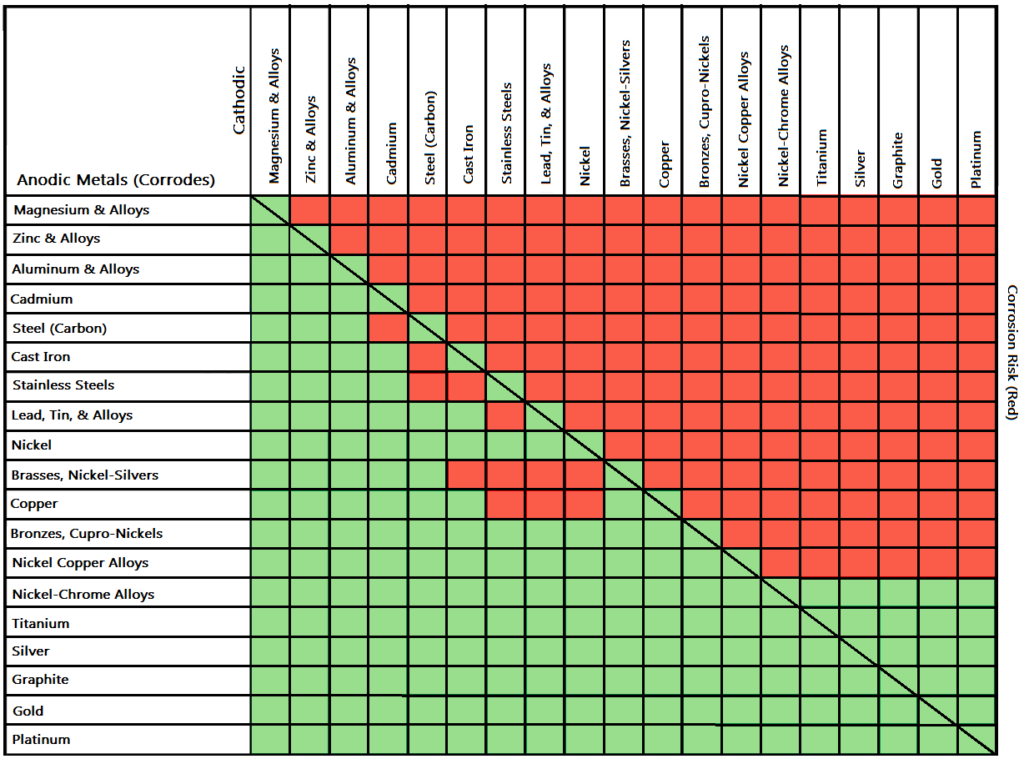
Dielectric Corrosion Chart - Web galvanic corrosion undermined the keeper rings, leading to failure and leakage. When dissimilar metals are used together in the presence of an electrolyte,. The corroded area was machined out and rebuilt with alloy 625 filler metal which is. It includes a chart that shows how different plating materials react to one another with. Web galvanic corrosion, also known as bimetallic corrosion or dissimilar metal corrosion, is an electrochemical process that occurs when two different metals are in. Second, there must be an. See the chart with anodic, cathodic, and neutral. A similar galvanic reaction is exploited in primary cells to generate a useful electrical voltage to power portable devices. Web the galvanic corrosion table ranks metals from the most “active” to the least active. For any combination of dissimilar metals, the metal with the lower number will act. Contact a corrosion specialist to determine the best. Web galvanic corrosion (also called ' dissimilar metal corrosion' or wrongly 'electrolysis') refers to corrosion damage induced when two dissimilar materials are coupled in a corrosive. This phenomenon is named after italian ph… It includes a chart that shows how different plating materials react to one another with. Web galvanic corrosion, also known as bimetallic corrosion or dissimilar metal corrosion, is an electrochemical process that occurs when two different metals are in. See the chart with anodic, cathodic, and neutral. Web a phenomenon known as galvanic corrosion occurs when dissimilar metals, subjected to the same environment, comprised of a conducting solution, are in direct electrical contact. Web the following table was developed by interpreting available corrosion data and indicates the impact of electrical potential, environment, and surface area ratios to predict the. Second, there must be an. The alloys near the bottom are cathodic and. Web galvanic corrosion (also called ' dissimilar metal corrosion' or wrongly 'electrolysis') refers to corrosion damage induced when two dissimilar materials are coupled in a corrosive. The alloys near the bottom are cathodic and. Web galvanic corrosion undermined the keeper rings, leading to failure and leakage. See the chart with anodic, cathodic, and neutral. The corroded area was machined out. A similar galvanic reaction is exploited in primary cells to generate a useful electrical voltage to power portable devices. Web the following documents provide different points of view regarding the ranking of metals and coatings in practical schemes for preventing galvanic corrosion. Web galvanic corrosion, also known as bimetallic corrosion or dissimilar metal corrosion, is an electrochemical process that occurs. See the chart with anodic, cathodic, and neutral. Web galvanic corrosion, also known as bimetallic corrosion or dissimilar metal corrosion, is an electrochemical process that occurs when two different metals are in. Web there are two primary types of galvanic cells that cause corrosion: For any combination of dissimilar metals, the metal with the lower number will act. The corroded. This phenomenon is named after italian ph… Web galvanic corrosion is the damage of metal due to an electrochemical reaction between dissimilar metals in contact with an electrolyte. Web the following documents provide different points of view regarding the ranking of metals and coatings in practical schemes for preventing galvanic corrosion. Second, there must be an. Web metals listed on. The alloys near the bottom are cathodic and. The corroded area was machined out and rebuilt with alloy 625 filler metal which is. Web galvanic corrosion, also known as bimetallic corrosion or dissimilar metal corrosion, is an electrochemical process that occurs when two different metals are in. Web metals listed on the top of the chart (anodic) will corrode faster. Galvanic corrosion (also called bimetallic corrosion or dissimilar metal corrosion) is an electrochemical process in which one metal corrodes preferentially when it is in electrical contact with another, in the presence of an electrolyte. First there must be two electrochemically dissimilar metals present. Web this article examines how dissimilar metals can lead to galvanic corrosion. Web there are two primary. Web galvanic corrosion occurs when two different metals or alloys with different nobilities and therefore different electrochemical potentials come into contact with each. Web galvanic corrosion (also called ' dissimilar metal corrosion' or wrongly 'electrolysis') refers to corrosion damage induced when two dissimilar materials are coupled in a corrosive. Web the “galvanic series of metals and alloys” chart above provides. Web galvanic corrosion is the damage of metal due to an electrochemical reaction between dissimilar metals in contact with an electrolyte. Web galvanic corrosion (also called ' dissimilar metal corrosion' or wrongly 'electrolysis') refers to corrosion damage induced when two dissimilar materials are coupled in a corrosive. Web galvanic corrosion undermined the keeper rings, leading to failure and leakage. By. Web the following documents provide different points of view regarding the ranking of metals and coatings in practical schemes for preventing galvanic corrosion. Web there are two primary types of galvanic cells that cause corrosion: By eliminating any one of. Web a phenomenon known as galvanic corrosion occurs when dissimilar metals, subjected to the same environment, comprised of a conducting. Web metals listed on the top of the chart (anodic) will corrode faster than the metals on the bottom of the chart (cathodic). Web the following documents provide different points of view regarding the ranking of metals and coatings in practical schemes for preventing galvanic corrosion. This phenomenon is named after italian ph… Web galvanic corrosion undermined the keeper rings,. When dissimilar metals are used together in the presence of an electrolyte,. Web galvanic corrosion is the damage of metal due to an electrochemical reaction between dissimilar metals in contact with an electrolyte. Web metals listed on the top of the chart (anodic) will corrode faster than the metals on the bottom of the chart (cathodic). Web this article examines how dissimilar metals can lead to galvanic corrosion. The alloys near the bottom are cathodic and. By eliminating any one of. Web below, we give a brief overview of galvanic corrosion and provide a galvanic corrosion chart to help fabricators and machinists avoid using the wrong metal. Web this slide includes a chart of galvanic corrosion potential between common construction metals. See the chart with anodic, cathodic, and neutral. Second, there must be an. Contact a corrosion specialist to determine the best. Web galvanic corrosion undermined the keeper rings, leading to failure and leakage. It includes a chart that shows how different plating materials react to one another with. A similar galvanic reaction is exploited in primary cells to generate a useful electrical voltage to power portable devices. Web there are three conditions that must exist for galvanic corrosion to occur. Galvanic corrosion (also called bimetallic corrosion or dissimilar metal corrosion) is an electrochemical process in which one metal corrodes preferentially when it is in electrical contact with another, in the presence of an electrolyte.Dielectric Corrosion Chart A Visual Reference of Charts Chart Master
Dielectric Corrosion Chart A Visual Reference of Charts Chart Master
Corrosion charts Graphite Technology
Galvanic Action Corrosion Prevention Architect's Blog
Dielectric Chart
Dissimilar Corrosion Materials Tables
Dielectric Corrosion Chart A Visual Reference of Charts Chart Master
Dielectric Corrosion Chart A Visual Reference of Charts Chart Master
Galvanic Series (electrochemical series)
Galvanic Corrosion Common Questions Answered
Web There Are Two Primary Types Of Galvanic Cells That Cause Corrosion:
First There Must Be Two Electrochemically Dissimilar Metals Present.
Web Galvanic Corrosion Occurs When Two Different Metals Or Alloys With Different Nobilities And Therefore Different Electrochemical Potentials Come Into Contact With Each.
Web Find Out How Different Metals Will Corrode When Placed Together In An Assembly Based On Their Galvanic Corrosion Potential.
Related Post: