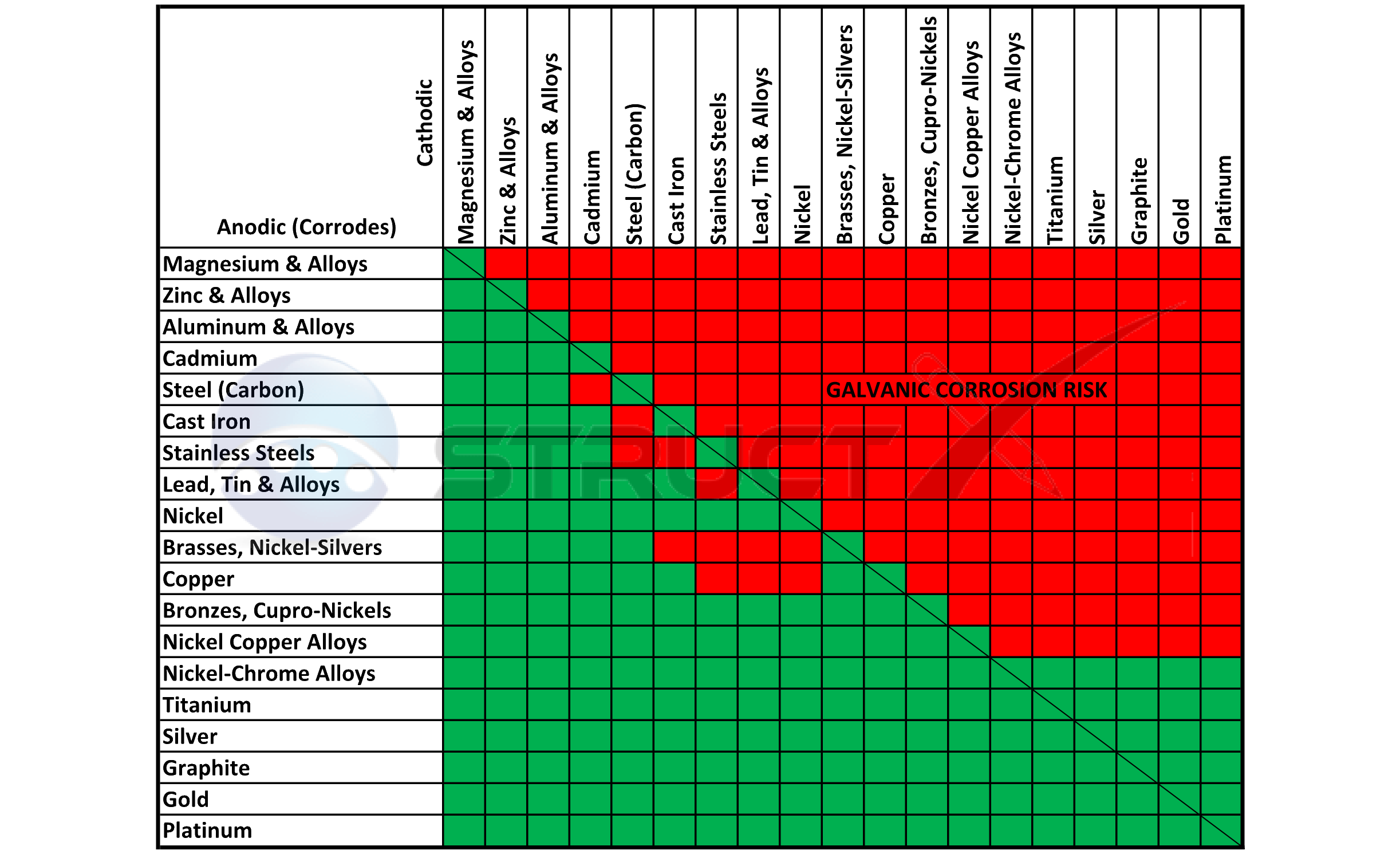
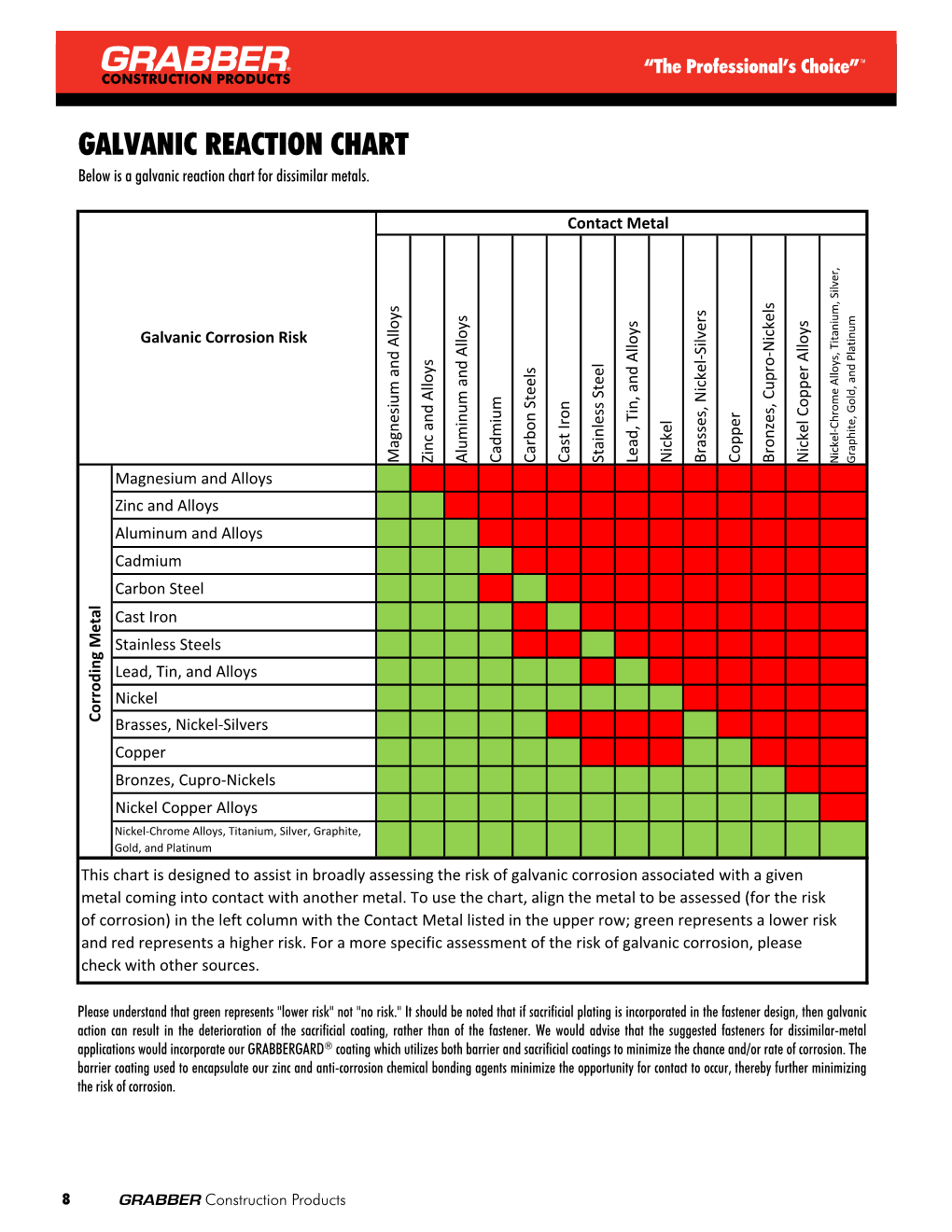
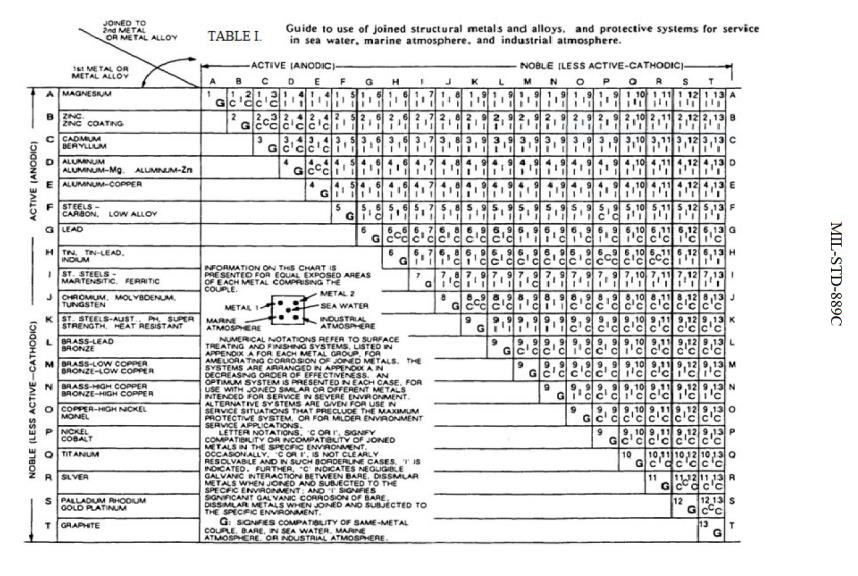
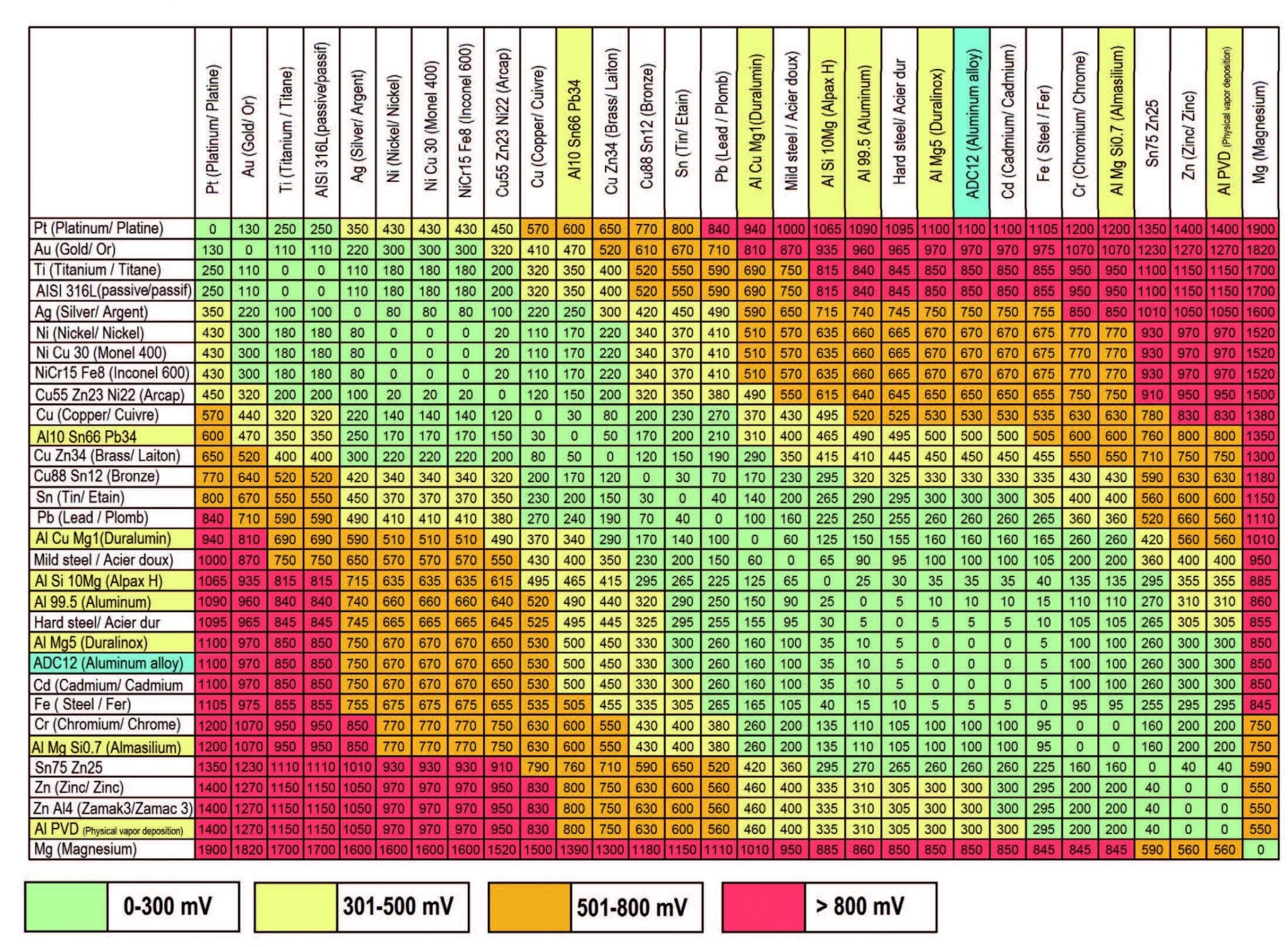
Dissimilar Metals Chart
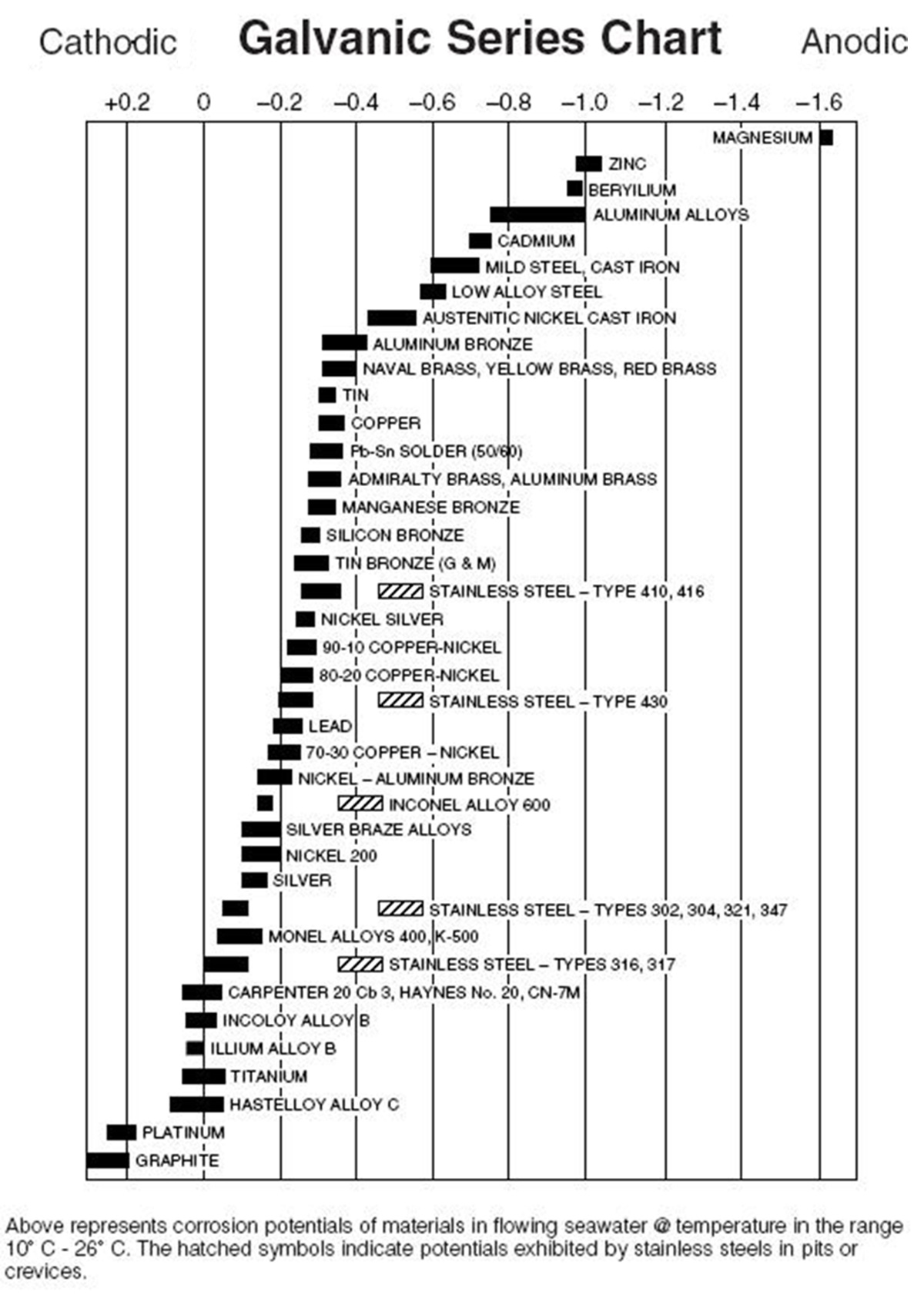
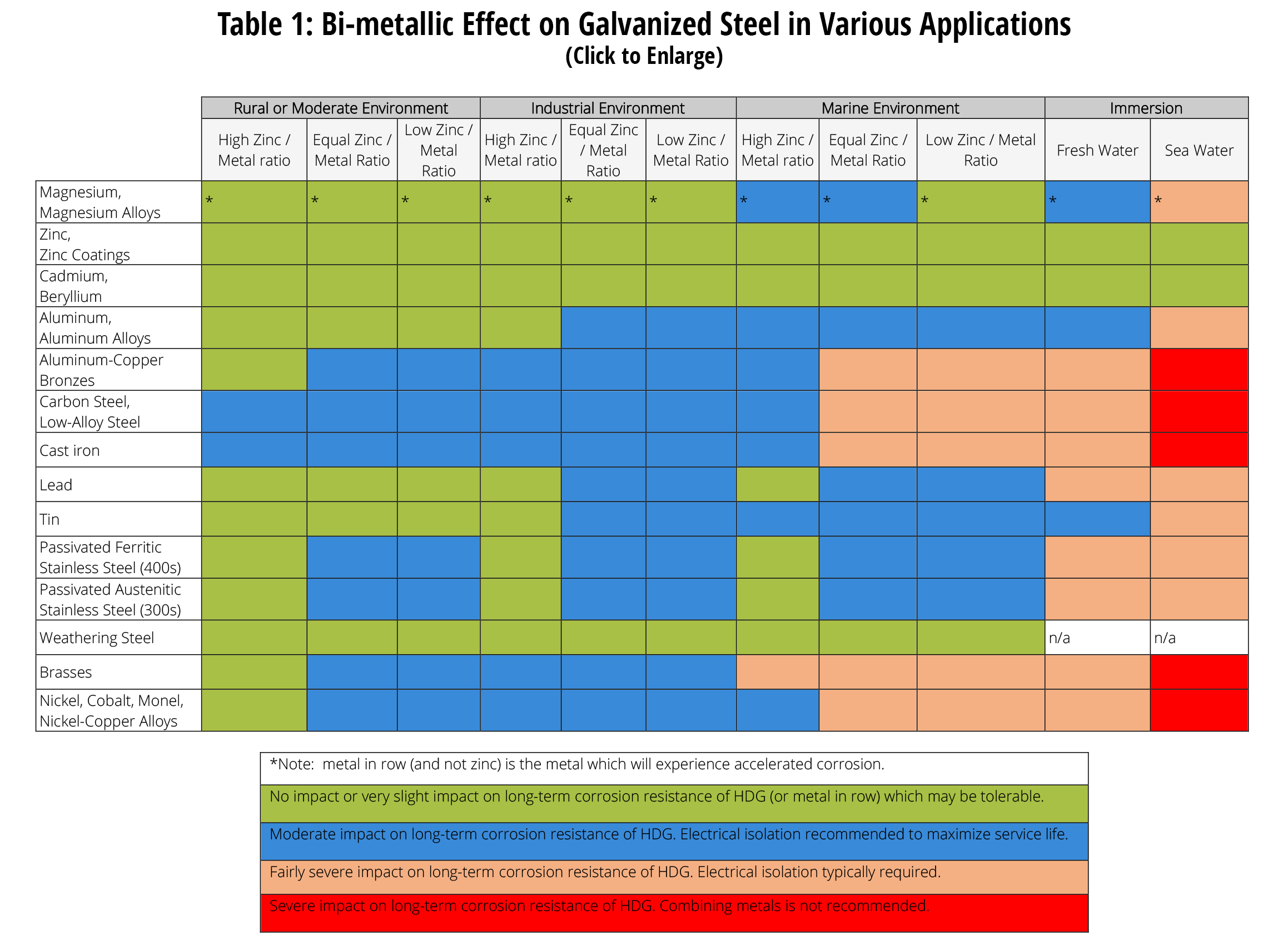
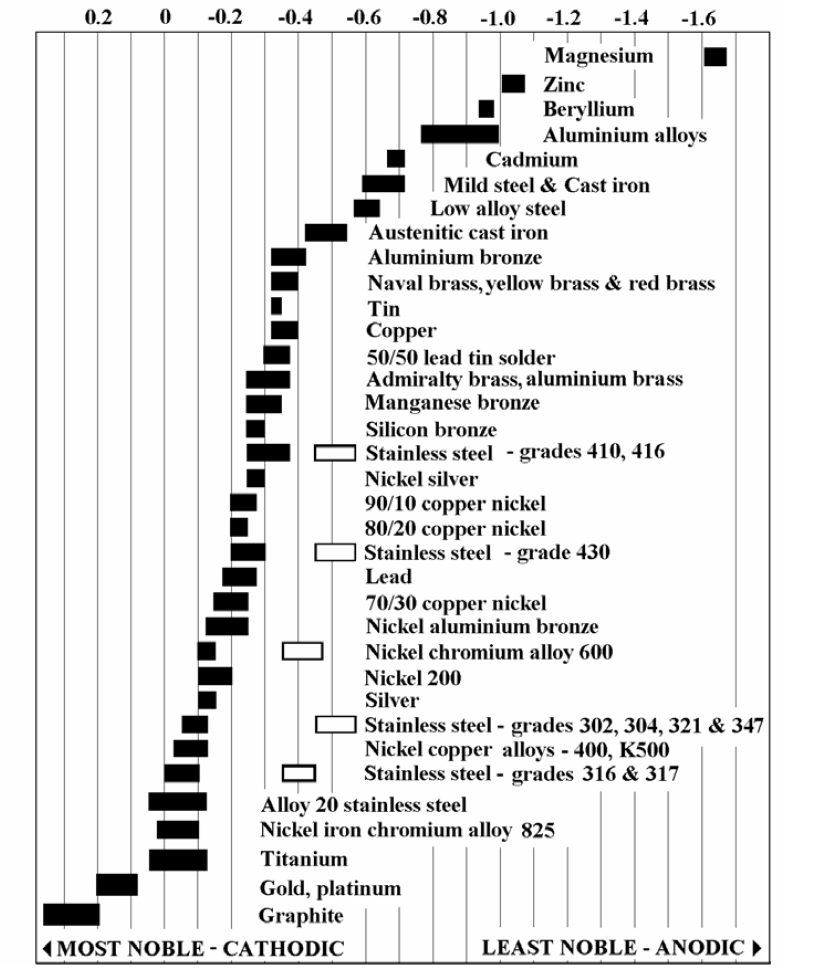
Dissimilar Metals Chart - Web this slide includes a chart of galvanic corrosion potential between common construction metals. There are three conditions that must exist for galvanic corrosion to occur. In this article, we'll look at an example to illustrate the use of the galvanic table. Web galvanic corrosion occurs when two dissimilar metals with different potentials are placed in electrical contact in an electrolyte. When working with copper or aluminum use antioxidant pastes. The cart to the left is galvanic series in flowing sea water. When dissimilar metals are used together in the presence of an electrolyte, separate them with a dielectric material such as insulation, paint or similar surface coating. Web below is a galvanic reaction chart for dissimilar metals. Web galvanic or dissimilar metal corrosion is electrochemical corrosion that occurs when one metal comes in contact with another material. Web the galvanic series chart below shows metals and their electrochemical voltage range (relative activity in flowing sea water). When dissimilar metals are used together in the presence of an electrolyte, separate them with a dielectric material such as insulation, paint or similar surface coating. Web galvanic corrosion occurs when two dissimilar metals with different potentials are placed in electrical contact in an electrolyte. Web this slide includes a chart of galvanic corrosion potential between common construction metals. Web galvanic corrosion potential is a measure of how dissimilar metals will corrode when placed against each other in an assembly. This chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given metal coming into contact with another metal. Web below is a galvanic reaction chart for dissimilar metals. The greater the potential difference is, the greater the tendency for corrosion. This chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given metal coming into contact with another metal. Web we are aa metals, inc, one of north america’s largest master distributors of quality aluminum and stainless products. Web the galvanic series chart below shows metals and their electrochemical voltage range (relative activity in flowing sea water). Electrolytic corrosion (electrolysis) occurs when dissimilar metals are in contact in the presence of an electrolyte, such as water (moisture) containing very small amounts of acid. This conversion resource can help you determine what metal gauge you may need. When working with copper or aluminum use antioxidant pastes. Web below, we give a brief overview of galvanic corrosion and provide. Web below, we give a brief overview of galvanic corrosion and provide a galvanic corrosion chart to help fabricators and machinists avoid using the wrong metal combinations. We also provide other helpful methods for avoiding galvanic corrosion. We also offer technical support including easy access to installation guides, product literature, technical bulletins and color charts. Web chart sheet metal gauge.. Web galvanic corrosion (some times called dissimilar metal corrosion) is the process by which the materials in contact with each other oxidizes or corrodes. A typical rule of thumb is that voltage differences of 0.2 volts or more suggest a galvanic corrosion risk. This chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with a. Web our extensive testing and independent certification program provide our customers access to over 40 florida product approvals on 11 different metal panel profiles. Weathertight warranties are also available. Web when design requires that dissimilar metals come in contact, galvanic compatibility can be managed by finishes and plating which protects the base materials from corrosion. Web this article examines how. Electrolytic corrosion (electrolysis) occurs when dissimilar metals are in contact in the presence of an electrolyte, such as water (moisture) containing very small amounts of acid. This type of accelerated corrosion between dissimilar metals is referred to as galvanic corrosion. The greater the potential difference is, the greater the tendency for corrosion. This chart is designed to assist in broadly. Web our extensive testing and independent certification program provide our customers access to over 40 florida product approvals on 11 different metal panel profiles. This type of accelerated corrosion between dissimilar metals is referred to as galvanic corrosion. In this article, we'll look at an example to illustrate the use of the galvanic table. The cart to the left is. There are three conditions that must exist for galvanic corrosion to occur. When working with copper or aluminum use antioxidant pastes. Web galvanic corrosion (also called bimetallic corrosion or dissimilar metal corrosion) is an electrochemical process in which one metal corrodes preferentially when it is in electrical contact with another, in the presence of an electrolyte. A typical rule of. You can also learn more about overcoming potentially compatibility issues between metals. In this article, we'll look at an example to illustrate the use of the galvanic table. Web we are aa metals, inc, one of north america’s largest master distributors of quality aluminum and stainless products. Web galvanic corrosion potential is a measure of how dissimilar metals will corrode. Web the susceptibility of different base metals to corrosion while in contact depends upon the difference between the contact potentials or the electromotive voltages of the metals involved. You can also learn more about overcoming potentially compatibility issues between metals. Web we are aa metals, inc, one of north america’s largest master distributors of quality aluminum and stainless products. This. We also offer technical support including easy access to installation guides, product literature, technical bulletins and color charts. There are three conditions that must exist for galvanic corrosion to occur. Web below is a galvanic reaction chart for dissimilar metals. This type of accelerated corrosion between dissimilar metals is referred to as galvanic corrosion. Web galvanic corrosion (also called bimetallic. Web galvanic or dissimilar metal corrosion is electrochemical corrosion that occurs when one metal comes in contact with another material. First there must be two electrochemically dissimilar metals present. The greater the potential difference is, the greater the tendency for corrosion. Web below is a galvanic reaction chart for dissimilar metals. A typical rule of thumb is that voltage differences of 0.2 volts or more suggest a galvanic corrosion risk. The cart to the left is galvanic series in flowing sea water. Weathertight warranties are also available. Web this article examines how dissimilar metals can lead to galvanic corrosion. Web read on to find out about what it is and how to use it to analyse the compatibility of joining metals. This chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given metal coming into contact with another metal. It includes a chart that shows how different plating materials react to one another with regard to their galvanic potential. In this article, we'll look at an example to illustrate the use of the galvanic table. • harsh environments, such as outdoors, high humidity, and salt environments. When dissimilar metals are used together in the presence of an electrolyte, separate them with a dielectric material such as insulation, paint or similar surface coating. There are three conditions that must exist for galvanic corrosion to occur. Web but when you step onto a jobsite, working with dissimilar metals turns into a whole new monster with dire potential consequences.Dissimilar joining of Al with steel? r/Welding
Galvanic Reaction Chart
Dissimilar Welding Chart Stainless Steel Transition Metals
Design Calculations of Lightning Protection Systems Part Fifteen
Dissimilar metal corrosion with chemical filmed (Alodine, Iridite
Galvanic Corrosion Chart Dissimilar Metals A Visual Reference of
Galvanic Corrosion Chart Dissimilar Metals
Dissimilar Metal Corrosion Chart
Dissimilar Metal Corrosion with… American Galvanizers Association
Dissimilar Metals Corrosion Chart
Web The Galvanic Series Chart Below Shows Metals And Their Electrochemical Voltage Range (Relative Activity In Flowing Sea Water).
Web Below, We Give A Brief Overview Of Galvanic Corrosion And Provide A Galvanic Corrosion Chart To Help Fabricators And Machinists Avoid Using The Wrong Metal Combinations.
Web We Are Aa Metals, Inc, One Of North America’s Largest Master Distributors Of Quality Aluminum And Stainless Products.
We Also Provide Other Helpful Methods For Avoiding Galvanic Corrosion.
Related Post: