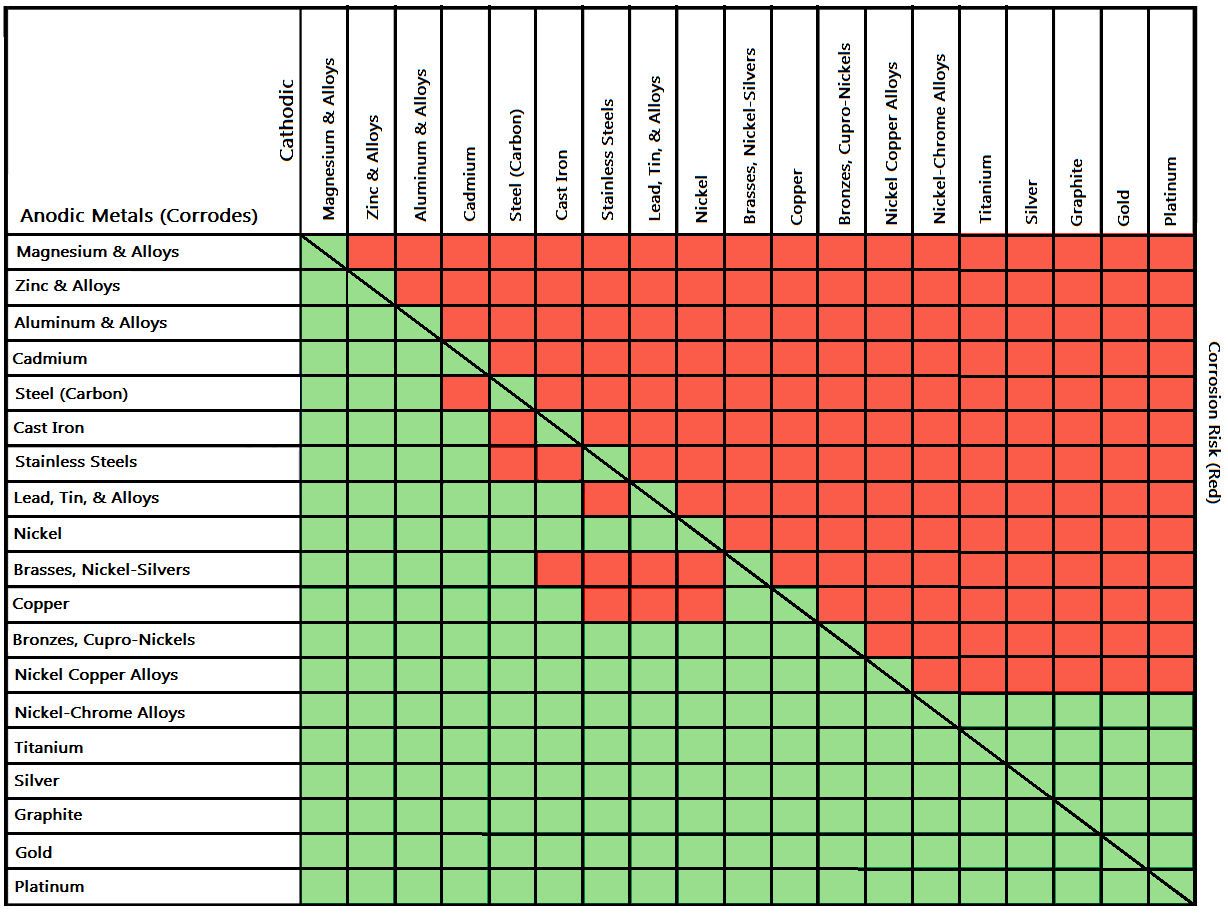
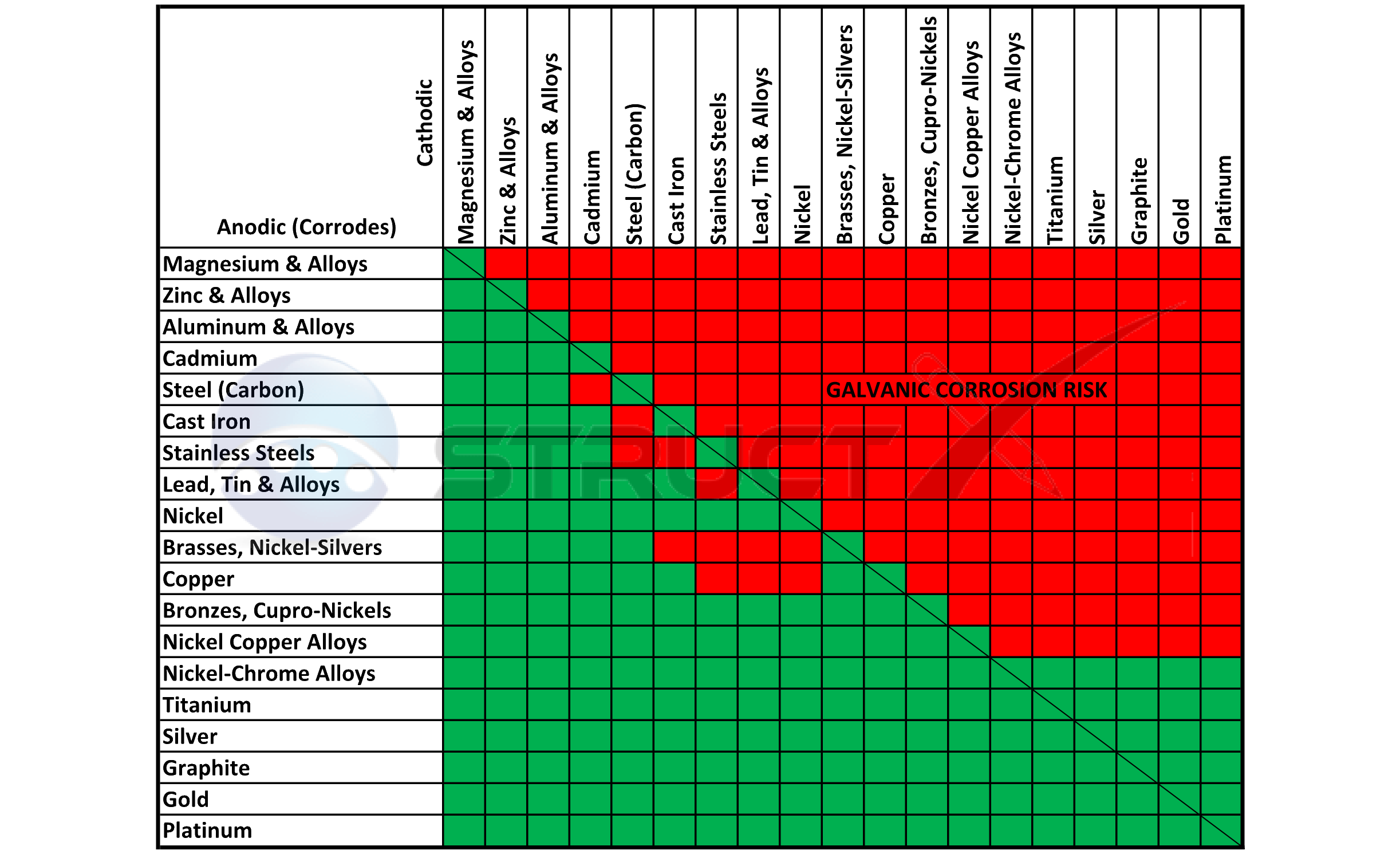
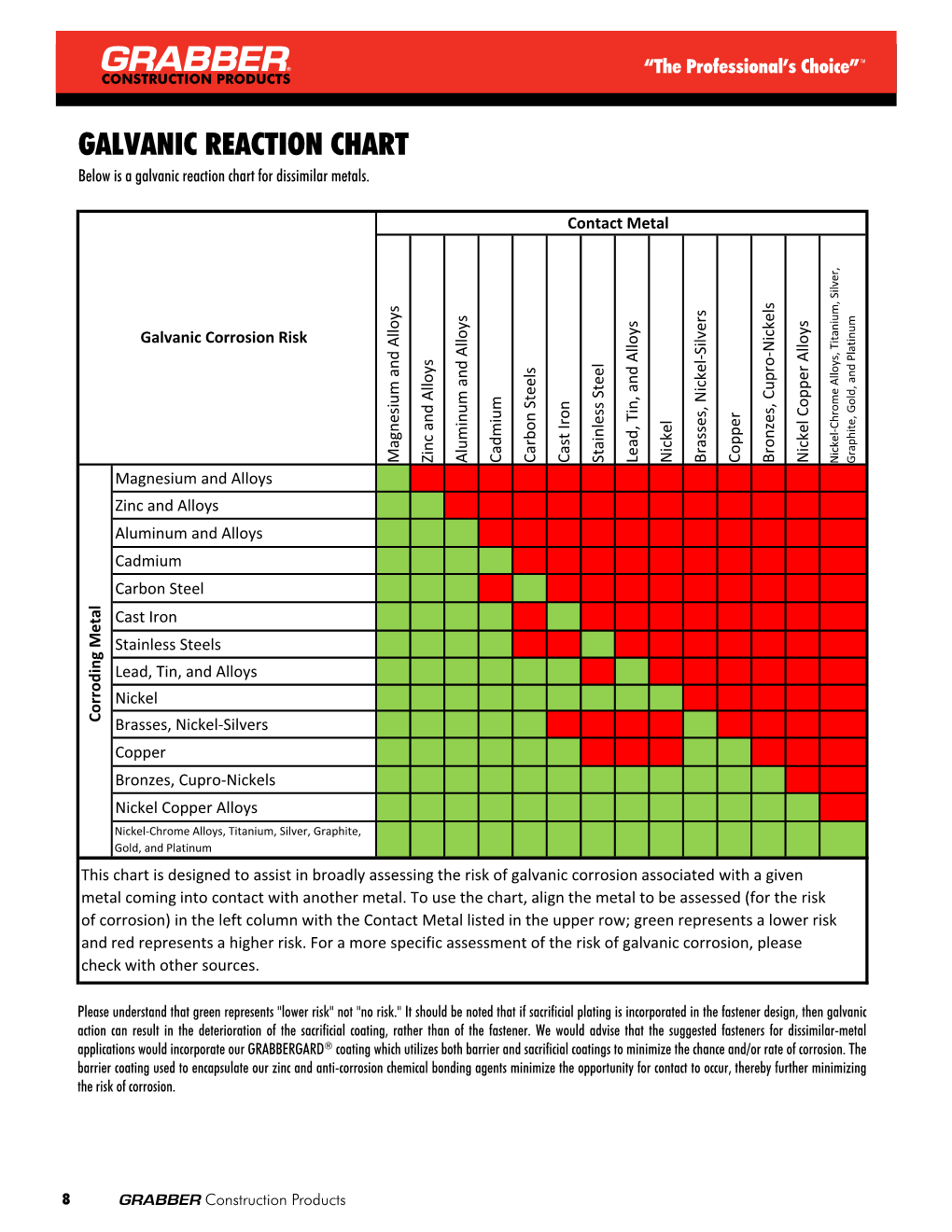
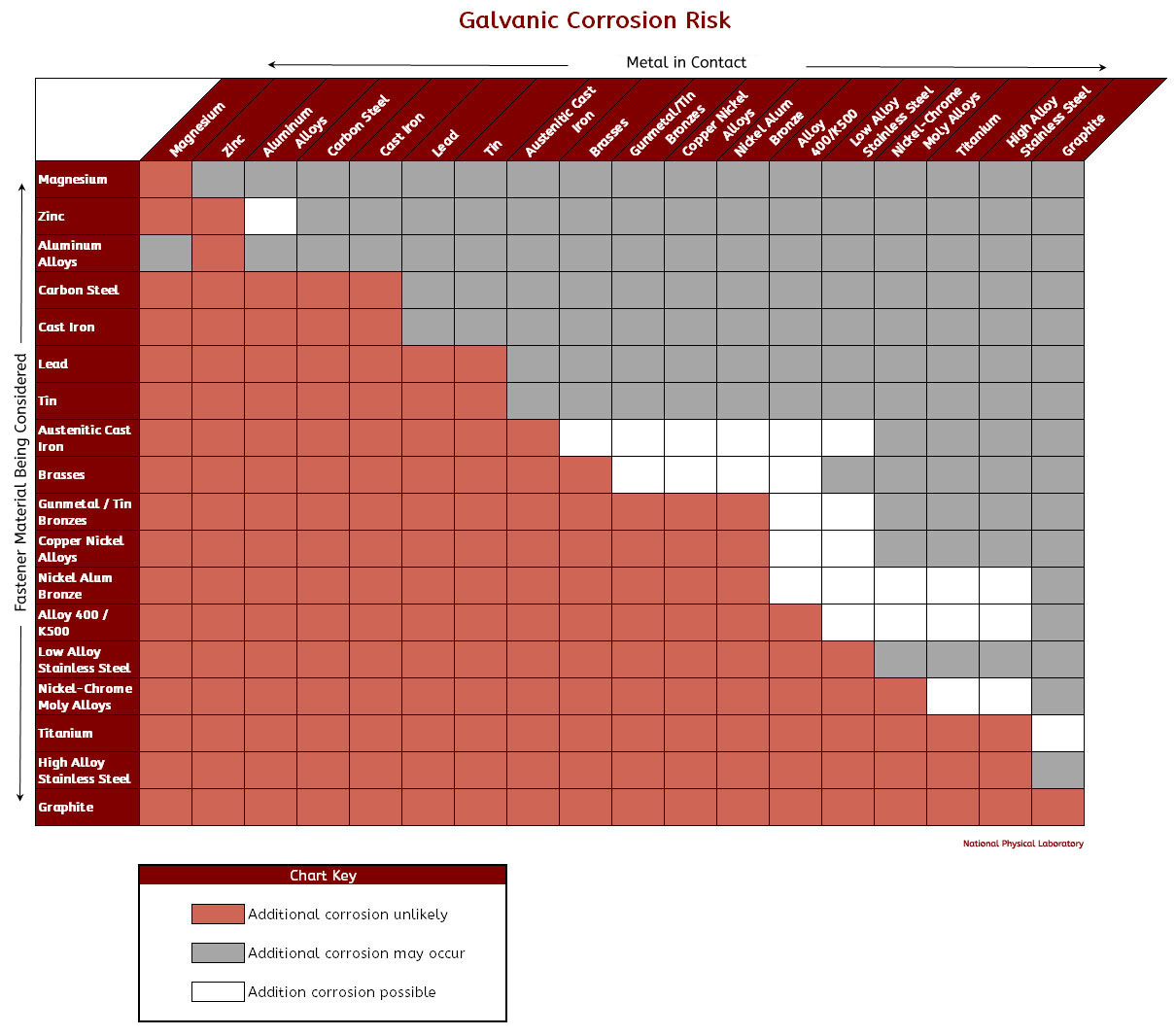
Galvanic Action Chart
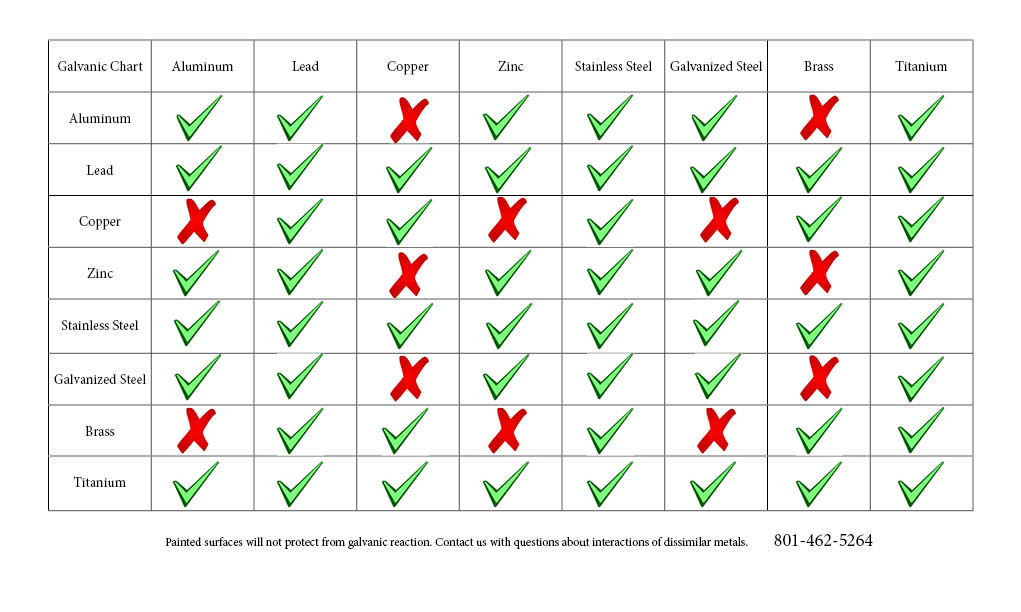
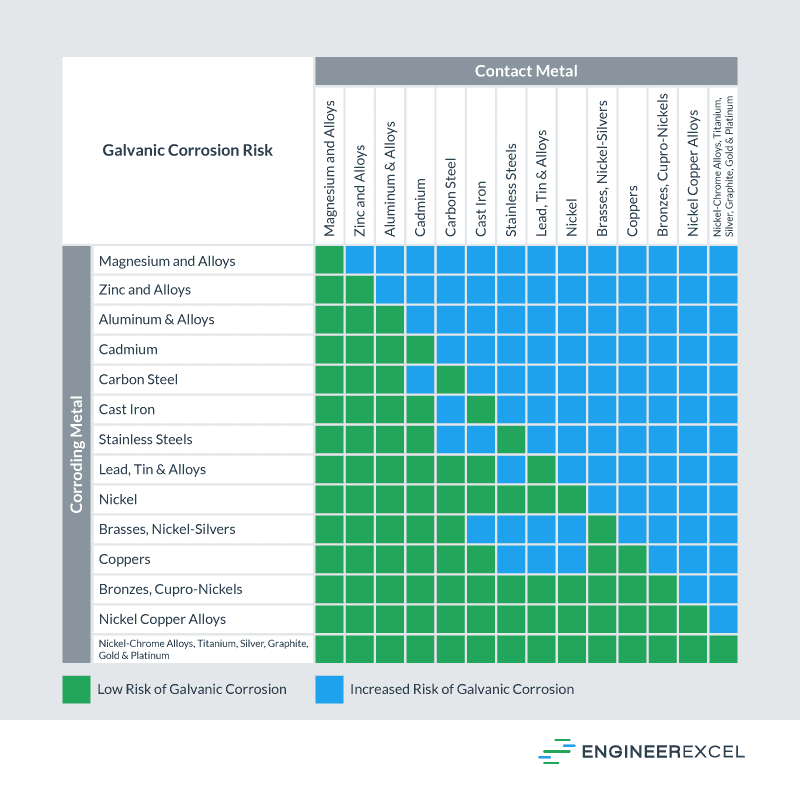
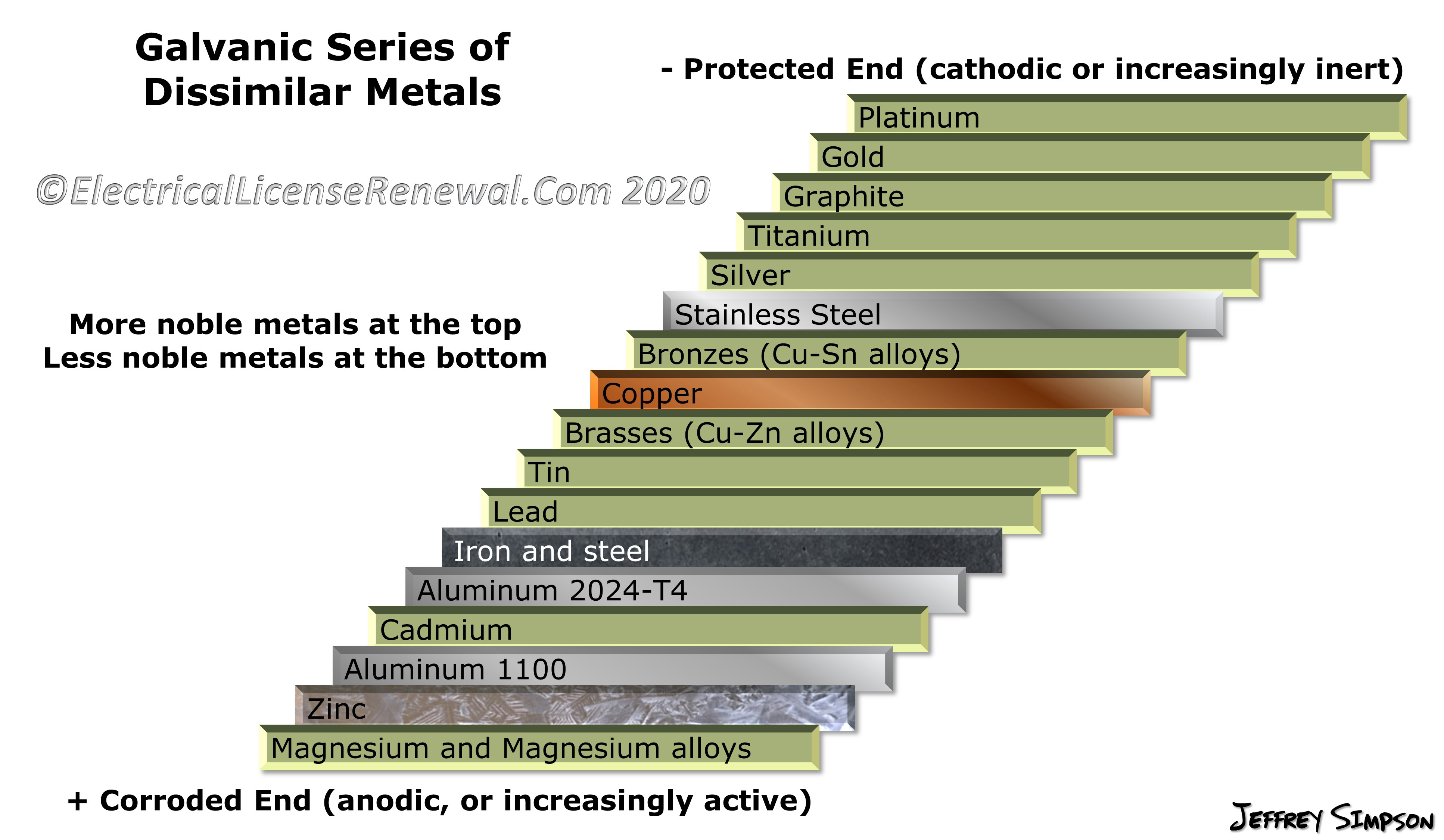
Galvanic Action Chart - Web the galvanic corrosion chart, also referred to as the galvanic series or compatibility chart, is a valuable tool used to assess the compatibility of different metal. Web galvanic corrosion (also called bimetallic corrosion or dissimilar metal corrosion) is an electrochemical process in which one metal corrodes preferentially when it is in electrical. Web below is a galvanic reaction chart for dissimilar metals. Though the order of metals in a. Web however, you can completely avoid galvanic corrosion by choosing matching metal anchors. Web read on to find out about what it is and how to use it to analyse the compatibility of joining metals. The closer together the material are on the chart to the right, the less. The below galvanic corrion chart or anodic index table shows anodic index for different materials. Web learn what galvanic action is, how it occurs, and how to prevent it in construction. Web the increased corrosion of the anode is called “galvanic corrosion.” galvanic corrosion is sometimes used to extend the life of materials (i.e. So, for example, choosing zinc on zinc would have the lowest risk for. Web electrolytic corrosion (electrolysis) occurs when dissimilar metals are in contact in the presence of an electrolyte, such as water (moisture) containing very small amounts of. The below galvanic corrion chart or anodic index table shows anodic index for different materials. Web read on to find out about what it is and how to use it to analyse the compatibility of joining metals. The most active metals in the galvanic corrosion chart, like aluminum, zinc, or. In this article, we'll look at an example to illustrate the use. Though the order of metals in a. The list begins with the more. Web there are four elements necessary for corrosion to occur in a galvanic cell: Web learn what galvanic action is, how it occurs, and how to prevent it in construction. The list begins with the more. Web however, you can completely avoid galvanic corrosion by choosing matching metal anchors. Web galvanic corrosion (some times called dissimilar metal corrosion) is the process by which the materials in contact with each other oxidizes or corrodes. The following galvanic table lists metals in the order of their relative activity in seawater environment. Web. Web to minimize galvanic corrosion, select fasteners based on their material compatibility with the substrates. We consider two metals compatible if their emf. Web galvanic corrosion (some times called dissimilar metal corrosion) is the process by which the materials in contact with each other oxidizes or corrodes. See a chart of metals and their electrochemical potentials, and examples. The closer. The list begins with the more. In this article, we'll look at an example to illustrate the use. Web below is a galvanic reaction chart for dissimilar metals. Web however, you can completely avoid galvanic corrosion by choosing matching metal anchors. This chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given metal. Web below is a galvanic reaction chart for dissimilar metals. The closer together the material are on the chart to the right, the less. Web below, we give a brief overview of galvanic corrosion and provide a galvanic corrosion chart to help fabricators and machinists avoid using the wrong metal. The list begins with the more. Web below is a. The following galvanic table lists metals in the order of their relative activity in seawater environment. Web the increased corrosion of the anode is called “galvanic corrosion.” galvanic corrosion is sometimes used to extend the life of materials (i.e. Web read on to find out about what it is and how to use it to analyse the compatibility of joining. Web below, we give a brief overview of galvanic corrosion and provide a galvanic corrosion chart to help fabricators and machinists avoid using the wrong metal. Web however, you can completely avoid galvanic corrosion by choosing matching metal anchors. Web galvanic corrosion (some times called dissimilar metal corrosion) is the process by which the materials in contact with each other. The following galvanic table lists metals in the order of their relative activity in seawater environment. Web electrolytic corrosion (electrolysis) occurs when dissimilar metals are in contact in the presence of an electrolyte, such as water (moisture) containing very small amounts of. In this article, we'll look at an example to illustrate the use. Web the galvanic corrosion table ranks. The following galvanic table lists metals in the order of their relative activity in seawater environment. Web the increased corrosion of the anode is called “galvanic corrosion.” galvanic corrosion is sometimes used to extend the life of materials (i.e. Web a chart depicting the galvanic series for some common metals in a frequently encountered conducting solution, seawater, is included in. Web galvanic corrosion (some times called dissimilar metal corrosion) is the process by which the materials in contact with each other oxidizes or corrodes. The following galvanic table lists metals in the order of their relative activity in seawater environment. Web read on to find out about what it is and how to use it to analyse the compatibility of. Web galvanic corrosion (some times called dissimilar metal corrosion) is the process by which the materials in contact with each other oxidizes or corrodes. Web read on to find out about what it is and how to use it to analyse the compatibility of joining metals. Web the galvanic corrosion table ranks metals from the most “active” to the least. Web learn what galvanic action is, how it occurs, and how to prevent it in construction. This chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given metal coming. Web electrolytic corrosion (electrolysis) occurs when dissimilar metals are in contact in the presence of an electrolyte, such as water (moisture) containing very small amounts of. Though the order of metals in a. The below galvanic corrion chart or anodic index table shows anodic index for different materials. Web view this chart of galvanic compatibility. The following galvanic table lists metals in the order of their relative activity in seawater environment. In this article, we'll look at an example to illustrate the use. Web below is a galvanic reaction chart for dissimilar metals. The closer together the material are on the chart to the right, the less. Web a chart depicting the galvanic series for some common metals in a frequently encountered conducting solution, seawater, is included in figure 1. Web below, we give a brief overview of galvanic corrosion and provide a galvanic corrosion chart to help fabricators and machinists avoid using the wrong metal. Web to minimize galvanic corrosion, select fasteners based on their material compatibility with the substrates. Web however, you can completely avoid galvanic corrosion by choosing matching metal anchors. Web read on to find out about what it is and how to use it to analyse the compatibility of joining metals. Web there are four elements necessary for corrosion to occur in a galvanic cell:Galvanic Corrosion Common Questions Answered
Galvanic Action Chart PDF
Galvanic Action Corrosion Prevention Architect's Blog
Galvanic Series (electrochemical series)
Galvanic Reaction Chart
Galvanic Reaction Chart All Points Fasteners
Galvanic Corrosion Chart
Galvanic Chart For Metals
Galvanic Corrosion Between Metals Chart
Galvanic Action Chart
The Most Active Metals In The Galvanic Corrosion Chart, Like Aluminum, Zinc, Or.
This Can Help You In The Selection Of The Best.
Web Galvanic Corrosion (Some Times Called Dissimilar Metal Corrosion) Is The Process By Which The Materials In Contact With Each Other Oxidizes Or Corrodes.
Web The Galvanic Corrosion Chart, Also Referred To As The Galvanic Series Or Compatibility Chart, Is A Valuable Tool Used To Assess The Compatibility Of Different Metal.
Related Post: