Polarity Chart For Solvents
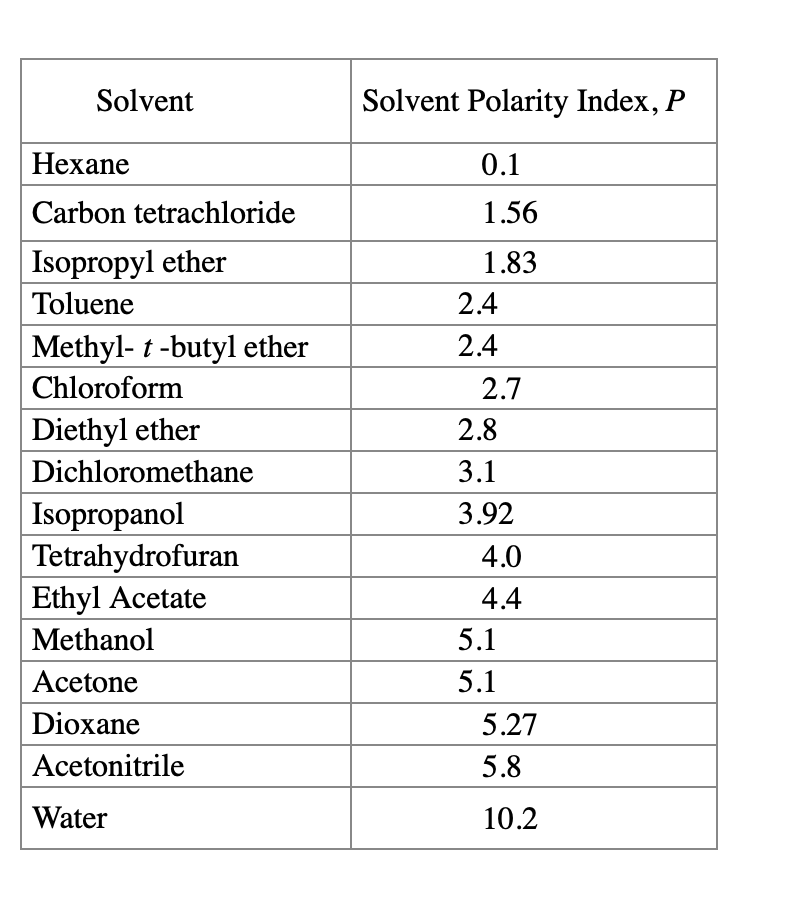
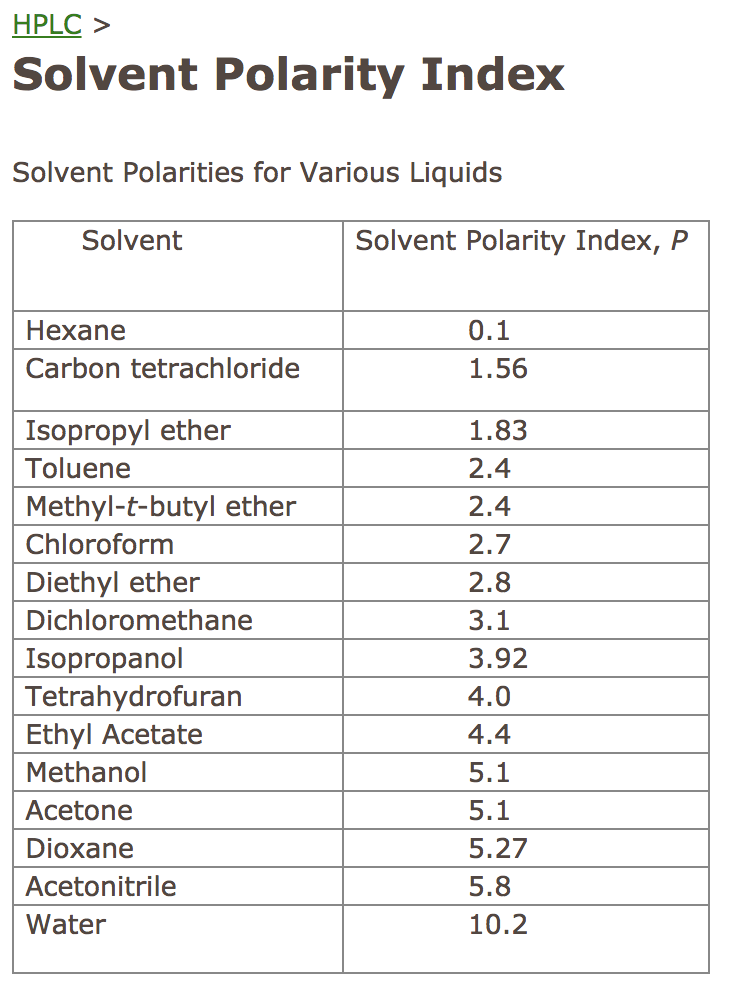
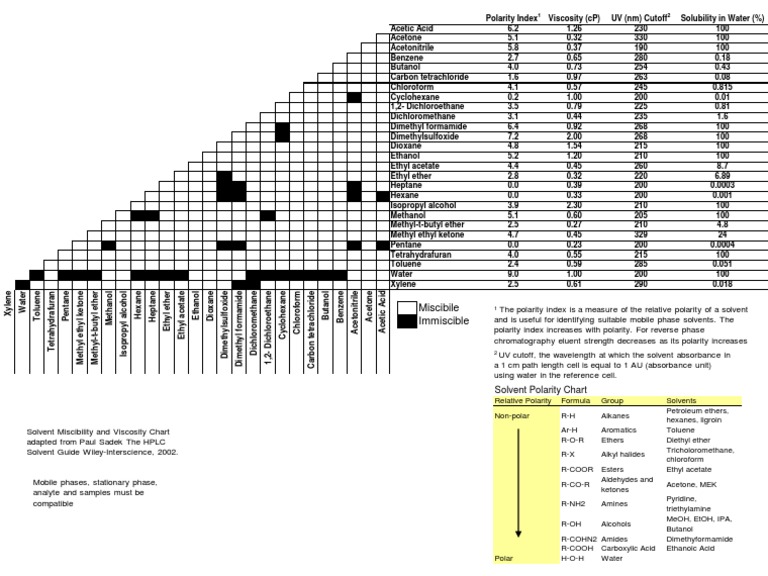
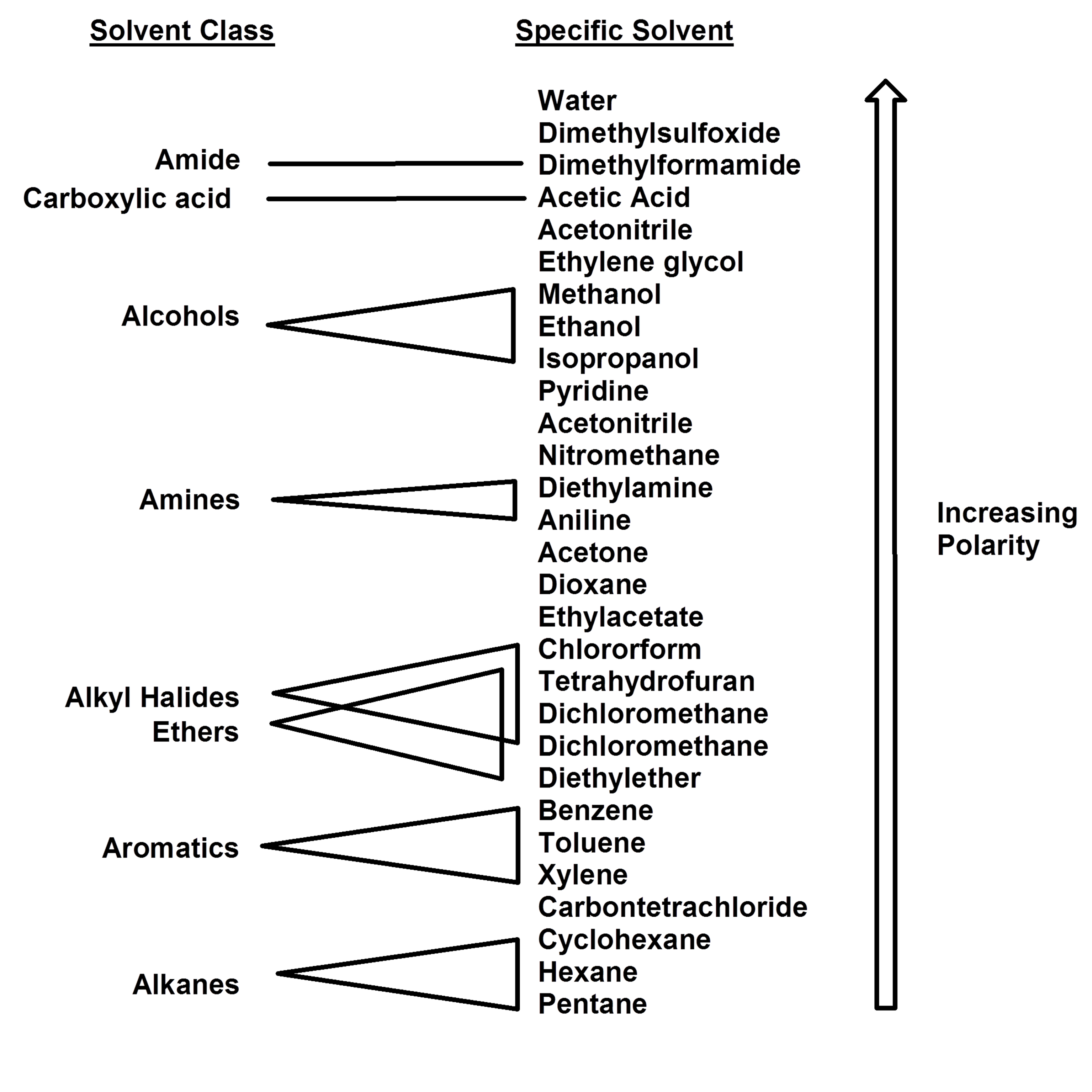
Polarity Chart For Solvents - 641 shows some of the most common crystallization solvents arranged by order of decreasing polarity going from top to bottom. Web 47 rows information on the properties of common solvents used in organic chemistry including boiling points, solubility, density, dielectric constants, and flash points. Web today, we will explore how polar organic solvents are, and will test what mixes with what. Web explain the concepts of polar covalent bonds and molecular polarity; Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline dimethylsulfoxide ethyl acetate. Some compounds are clearly very polar (e.g. Web table 10.1 on p. These ‘‘normalized selectivity’’ properties recognize three contributions of the solvent to. It also includes charts on relative solvent. Web the polarity of a covalent bond can be judged by determining the difference of the electronegativities of the two atoms involved in the covalent bond, as summarized. Assess the polarity of a molecule based on its bonding and structure Web it's easy enough to look up the miscibility of common laboratory solvents on charts like the one provided below; Water acetic acid ethyleneglycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diehylamine aniline dimethylsulfoxide ethylacetate. Demystifying synthetic organic chemistry since 2004. This column is based on one of the most widely used polar phases, carbowax™ 20m, and is a polar column suitable for analyses of solvents, fatty acid. It also includes charts on relative solvent. Web the document lists various solvents and provides their polarity index, viscosity, uv cutoff wavelength, solubility in water, and miscibility. Web polarity index (p1) defined as a measure of the ability of the solvent to interact with various test solutes. Common solvents arranged from the least polar to the most polar Web table 10.1 on p. Web because polar solvents stabilize the ground state more than the excited state, the change in energy increases with the polarity of the solvent. Web 1 the polarity index is a measure of the relative polarity of a solvent and is useful for identifying suitable mobile phase solvents. The values for relative polarity are normalized from measurements of solvent shifts. Web the polarity of a covalent bond can be judged by determining the difference of the electronegativities of the two atoms involved in the covalent bond, as summarized. Assess the polarity of a molecule based on its bonding and structure Solvents which are close to each. Web 23 rows solvent polarity; Web burdick & jackson solvents are arranged in order. P1 increases with solvent polarity. Web the polarity of a covalent bond can be judged by determining the difference of the electronegativities of the two atoms involved in the covalent bond, as summarized. Web 24 rows polarity indexes of solvents which are commonly used for sec analysis is. Assess the polarity of a molecule based on its bonding and structure. Web properties of solvents used in organic chemistry including mp, bp, desnity, water solubiity, polarity viscosity, dipole moment, dielectric constant Solvents which are close to each. Web polarity index (p1) defined as a measure of the ability of the solvent to interact with various test solutes. Web 24 rows polarity indexes of solvents which are commonly used for sec analysis. P1 increases with solvent polarity. The values for relative polarity are normalized from measurements of solvent shifts of absorption spectra and were extracted from christian reichardt,. Web it's easy enough to look up the miscibility of common laboratory solvents on charts like the one provided below; Web burdick & jackson solvents are arranged in order of increasing polarity index, a. Web properties of solvents used in organic chemistry including mp, bp, desnity, water solubiity, polarity viscosity, dipole moment, dielectric constant Web burdick & jackson solvents are arranged in order of increasing polarity index, a relative measure of the degree of interaction of the solvent with various polar test solutes. Web because polar solvents stabilize the ground state more than the. Web the polarity of a covalent bond can be judged by determining the difference of the electronegativities of the two atoms involved in the covalent bond, as summarized. Web because polar solvents stabilize the ground state more than the excited state, the change in energy increases with the polarity of the solvent. Web 1 the polarity index is a measure. However, novel and new substances or solvent combinations are. Web explain the concepts of polar covalent bonds and molecular polarity; Common solvents arranged from the least polar to the most polar Some compounds are clearly very polar (e.g. Demystifying synthetic organic chemistry since 2004. Web it's easy enough to look up the miscibility of common laboratory solvents on charts like the one provided below; Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline dimethylsulfoxide ethyl acetate. 641 shows some of the most common crystallization solvents arranged by order of decreasing polarity going from top to bottom. Web explain the concepts. Web 47 rows information on the properties of common solvents used in organic chemistry including boiling points, solubility, density, dielectric constants, and flash points. It also includes charts on relative solvent. The polarity index increases with polarity. Assess the polarity of a molecule based on its bonding and structure Common solvents arranged from the least polar to the most polar Demystifying synthetic organic chemistry since 2004. Solvents which are close to each. Web burdick & jackson solvents are arranged in order of increasing polarity index, a relative measure of the degree of interaction of the solvent with various polar test solutes. This page uses frames, but your browser doesn't support them. Web 1 the polarity index is a measure of the relative polarity of a solvent and is useful for identifying suitable mobile phase solvents. Web polarity index (p1) defined as a measure of the ability of the solvent to interact with various test solutes. Common solvents arranged from the least polar to the most polar Web it's easy enough to look up the miscibility of common laboratory solvents on charts like the one provided below; This column is based on one of the most widely used polar phases, carbowax™ 20m, and is a polar column suitable for analyses of solvents, fatty acid. Web properties of solvents used in organic chemistry including mp, bp, desnity, water solubiity, polarity viscosity, dipole moment, dielectric constant However, novel and new substances or solvent combinations are. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline dimethylsulfoxide ethyl acetate. Web the polarity of a covalent bond can be judged by determining the difference of the electronegativities of the two atoms involved in the covalent bond, as summarized. It also includes charts on relative solvent. Web the document lists various solvents and provides their polarity index, viscosity, uv cutoff wavelength, solubility in water, and miscibility. These ‘‘normalized selectivity’’ properties recognize three contributions of the solvent to.Polarity Chart Of Solvents
Polarity Chart Of Solvents
organic solvent polarity chart Bamil
Solvent Polarity Chart
Solved Determine the solvent polarity index for each HPLC
Solved Determine the solvent polarity index for the
Chemistry Solvent Characteristics CTG Clean
Solvent Miscibility and Polarity Chart
Polarity Of Solvents Chart
Solvent Polarity Chart A Visual Reference of Charts Chart Master
641 Shows Some Of The Most Common Crystallization Solvents Arranged By Order Of Decreasing Polarity Going From Top To Bottom.
The Polarity Index Increases With Polarity.
Web 24 Rows Polarity Indexes Of Solvents Which Are Commonly Used For Sec Analysis Is.
Some Compounds Are Clearly Very Polar (E.g.
Related Post: